Preoperative anemia is a common finding. Preoperative allogeneic transfusion, iron therapy, vitamin supplementation and erythropoietin therapy are the current management strategies for preoperative anemia. Previous reviews regarding erythropoietin were limited to specialties, provided little evidence regarding the benefits and risks of erythropoietin in managing preoperative anemia and included non-anemic patients. The purpose of our systematic review was to determine the role of erythropoietin solely in preoperatively anemic patients and to investigate the complications of this treatment modality to produce a guideline for preoperative management of anemic patients for all surgical specialties. The PubMed/Medline, Google Scholar, and Cochrane Library were searched for randomized trials evaluating the efficacy of erythropoietin in preoperative anemia. The risk ratio (RR) and standardized mean difference (SMD) was used to pool the estimates of categorical and continuous outcomes, respectively. Allogeneic transfusion and complications and the 90-day mortality were the primary outcomes, while the postoperative change in hemoglobin, bleeding in milliliters and the number of red blood cell (RBC) packs transfused were the secondary outcomes. Results: Eight studies were included, comprising 734 and 716 patients in the erythropoietin group and non-erythropoietin group, respectively. The pooled estimate by RR for allogeneic transfusion was 0.829 (p = 0.049), while complications and the 90-day mortality were among the 1,318 (p = 0.18) patients. Conclusion: Preoperative erythropoietin provides better outcomes, considering the optimization of preoperative anemia for elective surgical procedures. The benefits of erythropoietin are significantly higher, compared to the control group, while the risks remain equivocal in both groups. We recommend preoperative erythropoietin in anemic patients.
Anemia is a common finding in preoperative patients.1 It is defined as a hemoglobin level of less than 13 g/dl in adult males and less than 12 g/dl in adult non-pregnant females.2 Preoperative anemia is prevalent among 30–40% of the patients due for major surgery. It affects 54.4% of cardiac surgery patients and 39% of non-cardiac surgery patients.3,4 Multiple etiologies can predispose a person to anemia, with the main causes being nutritional deficiency anemia, anemia of chronic disease (ACD), repeated phlebotomies, dilutional anemia and bone marrow suppression due to sulfur drugs, chemotherapy and radiotherapy.5–8 Iron deficiency anemia (IDA) is reported to be the most common cause of anemia in preoperative patients, affecting 33% of the population.9 Moreover, previous research has stated that IDA, ACD and anemia of unexplained origin is amply present among surgical patients, as compared to other etiologies.10,11
Anemia is a serious concern that can adversely impact the outcome of surgical procedures.6 It leads to increased postoperative mortality and morbidities, such as respiratory and renal failure, infective complications and cardiovascular events.12,13 A 30-day risk of patient mortality is directly proportional to the decrease in the preoperative hemoglobin concentration, especially when its level is under 6 g/dl.14 However, preoperative anemia is a modifiable risk factor that needs to be effectively diagnosed, evaluated and managed. In major surgeries, for example, related to gynecology, orthopedics and cardiology, there is a massive risk for intraoperative blood loss, which could prove to be life-threatening if the hemoglobin concentration has not been adequately corrected preoperatively.13
The preoperative allogeneic blood transfusion is commonly performed to manage moderate to severely anemic patients.15 A total of 40–70% of all red blood cell (RBC) packs are transfused to surgical patients.7 Though a rapid procedure, it may prolong hospital stay and increase healthcare costs, as well as predispose the patient to transfusion reactions, postoperative hospital-acquired infections and immunological and thrombotic complications.16,17 There has been research that associated the RBC transfusion with adverse renal, cardiac and neurological outcomes, while the cost of screening blood products is a major issue for developing nations.15 The above-mentioned negative outcomes and the declination of blood transfusion by Jehovah’s Witnesses make it a less favorable option for anemia management. Iron supplementation is another widely utilized method in the management of preoperative anemia. Exogenous iron can be administered orally or parenterally, based on the patient adherence and the time when surgery is due, but the method produces little or no effect in ACD and malignancy.9 In addition, erythropoietin (EPO) therapy is another of the latest strategies used for the optimization of preoperative anemia. The EPO receptor-stimulating agents are recombinant forms of EPO that are injected to stimulate the production of red blood cells. They have been successfully used to treat anemia due to chronic disease and malignancy and IDA patients who do not respond to iron therapy alone in a short span of time.
Two previous reviews highlighted that preoperative optimization of anemia, using iron therapy and/or EPO therapy, is essential before the orthopedic procedure to avoid blood transfusions.18,19 Moreover, in their review, Cho et al. concluded that preoperative EPO used in surgical patients resulted in a significant reduction in perioperative blood transfusion.17 However, they were limited to certain specialties, such as the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS), recommended preoperative EPO in their guidelines for cardiac surgeries.20 The reviews lacked comparisons of the complications of the EPO treatment. The purpose of our systematic review and meta-analysis was to determine the probability of allogenic transfusion after the EPO in preoperatively anemic patients and to investigate the risks of this treatment modality.
Materials and methodsStrategy“Preferred reporting items for systematic reviews and meta-analysis (PRISMA)” was used to obtain research regarding outcomes of preoperative erythropoietin (EPO). The literature available was assessed by its title, abstract and, finally, full texts for the rendering of quality assessment scores.
DatabaseThe PubMed/Medline, Google Scholar and Cochrane library were systematically searched from 1990 to 2019 with the words “erythropoietin”, “transfusion” and “preoperative anemia” in different combinations for randomized trials in English on the human specimen. References of included trials were also checked for eligible studies.
Inclusion and exclusion criteriaThe inclusion and exclusion criteria were set after discussions among the authors. Only randomized trials that involved specified outcomes for EPO in anemic and non-anemic patients were included. The anemic participants, as well as the non-anemic, included in trials could not have any other associated hematological diseases, such as bone marrow disorders, hemolytic disorders or hemoglobinopathies. The intervention should be preoperative EPO, with or without other pharmacological therapy, such as iron therapy, folic acid and vitamin B12, to correct nutritional deficiencies. However, trials were excluded if the adjuvant pharmacological therapy could have predisposed the patient to bone marrow suppression, hemolysis or nutritional deficiencies. The control group was comprised of participants receiving either no intervention (placebo) or iron therapy alone. Participants in the placebo group were given normal salines or dextrose water, instead of EPO, on schedules similar to those of the EPO group, while participants in the group receiving iron therapy alone were given iron supplementation in the form of injectable iron sucrose or carboxymaltose. The control group participants with similar pathologies were obligatorily diagnosed and the surgical techniques they were treated with remained the same as those performed in the EPO group. Candidates in the control group could not have received EPO for any disorder, nor donated blood within the previous three months.
The studies were excluded if they included patients with bleeding diathesis, a history of EPO use, transfusion or iron therapy within the previous three months or the long-term use of bone marrow suppressants. The exclusion criteria also excluded studies where EPO was used to support preoperative autologous transfusion or other blood management strategies. Poor quality trials, letters, short communications, commentaries, editorials, case reports, cohort studies, cross-sectional studies, conference papers, proceedings and personal communications were excluded. The corresponding author of this article contacted the authors of trials to sort out any ambiguities within their trials before exclusion, in the case of no response or unsatisfactory response.
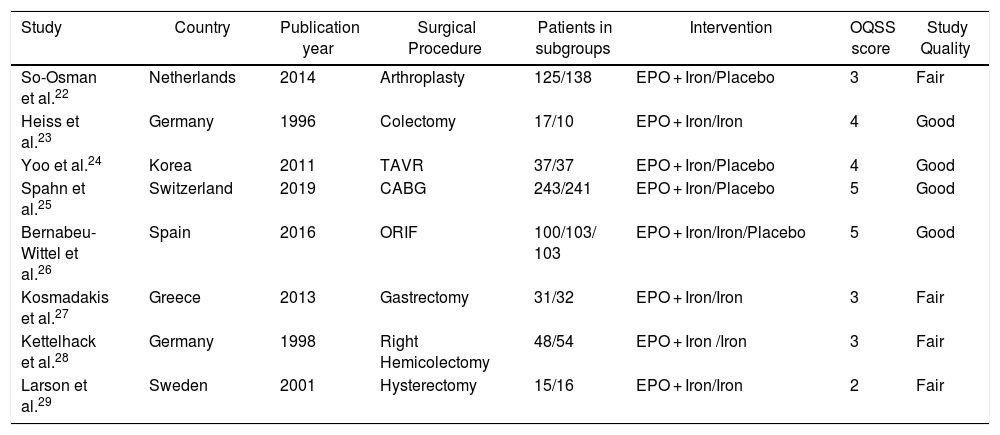
Risk of bias and quality assessmentThree authors (M.H.H, O.N. and S.M.E.A) scored the research independently with the quality assessment checklist for methodological quality by the “Oxford quality scoring system (OQSS)”21 for randomized trials. For the Oxford quality scoring system, a score of 5 or 4 suggests a good quality trial; 3 or 2 suggests a fair quality trial while 1 or 0 signifies a poor quality study. Any disagreements were resolved through internal discussion among the authors. An expert hematologist and anesthetist were involved if disagreements could not be resolved after discussions among authors. The assessment of the risk of bias by the OQSS score is shown in Table 1.
Characteristics of included studies.
| Study | Country | Publication year | Surgical Procedure | Patients in subgroups | Intervention | OQSS score | Study Quality |
|---|---|---|---|---|---|---|---|
| So-Osman et al.22 | Netherlands | 2014 | Arthroplasty | 125/138 | EPO + Iron/Placebo | 3 | Fair |
| Heiss et al.23 | Germany | 1996 | Colectomy | 17/10 | EPO + Iron/Iron | 4 | Good |
| Yoo et al.24 | Korea | 2011 | TAVR | 37/37 | EPO + Iron/Placebo | 4 | Good |
| Spahn et al.25 | Switzerland | 2019 | CABG | 243/241 | EPO + Iron/Placebo | 5 | Good |
| Bernabeu-Wittel et al.26 | Spain | 2016 | ORIF | 100/103/ 103 | EPO + Iron/Iron/Placebo | 5 | Good |
| Kosmadakis et al.27 | Greece | 2013 | Gastrectomy | 31/32 | EPO + Iron/Iron | 3 | Fair |
| Kettelhack et al.28 | Germany | 1998 | Right Hemicolectomy | 48/54 | EPO + Iron /Iron | 3 | Fair |
| Larson et al.29 | Sweden | 2001 | Hysterectomy | 15/16 | EPO + Iron/Iron | 2 | Fair |
OQSS scoring system: Randomization without method = 1 point; Randomization with method = 2 points (Deduct 1 point if inappropriate randomization method); Blinding without method = 1 point; Blinding with method = 2 points (Deduct 1 point if inappropriate blinding method), and; Withdrawal with reasons = 1 point.
OQSS score: 4–5 = Good, 2–3 = Fair.
EPO: Erythropoietin; TAVR: Transcatheter aortic valve replacement; CABG: Coronary artery bypass grafting; ORIF: Open reduction and internal fixation.
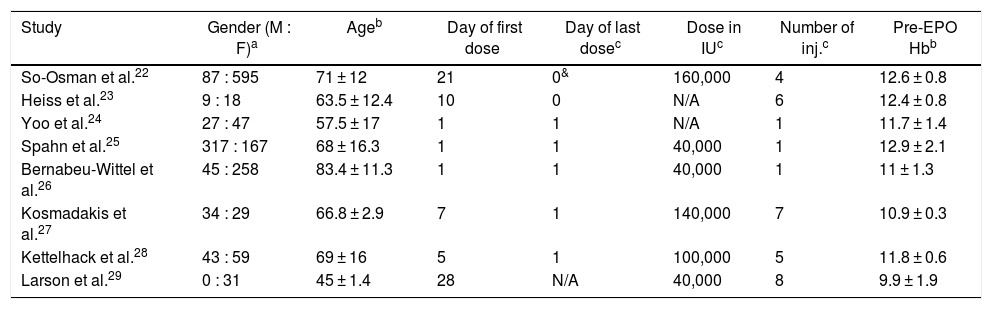
The following information was extracted from each study by three authors (M.H.H, O.N. and S.M.E.A): year of publication, country of the study, study design, patient characteristics, intervention in each subgroup, elective surgical procedure, male to female ratio, age, day of the first dose, day of the last dose, dose of EPO administered, number of EPO injections administered and preoperative hemoglobin (Hb) in gm/dl. Tables 1 and 2 show the extracted data for the studies included.
Characteristics of participants included in studies.
| Study | Gender (M : F)a | Ageb | Day of first dose | Day of last dosec | Dose in IUc | Number of inj.c | Pre-EPO Hbb |
|---|---|---|---|---|---|---|---|
| So-Osman et al.22 | 87 : 595 | 71 ± 12 | 21 | 0& | 160,000 | 4 | 12.6 ± 0.8 |
| Heiss et al.23 | 9 : 18 | 63.5 ± 12.4 | 10 | 0 | N/A | 6 | 12.4 ± 0.8 |
| Yoo et al.24 | 27 : 47 | 57.5 ± 17 | 1 | 1 | N/A | 1 | 11.7 ± 1.4 |
| Spahn et al.25 | 317 : 167 | 68 ± 16.3 | 1 | 1 | 40,000 | 1 | 12.9 ± 2.1 |
| Bernabeu-Wittel et al.26 | 45 : 258 | 83.4 ± 11.3 | 1 | 1 | 40,000 | 1 | 11 ± 1.3 |
| Kosmadakis et al.27 | 34 : 29 | 66.8 ± 2.9 | 7 | 1 | 140,000 | 7 | 10.9 ± 0.3 |
| Kettelhack et al.28 | 43 : 59 | 69 ± 16 | 5 | 1 | 100,000 | 5 | 11.8 ± 0.6 |
| Larson et al.29 | 0 : 31 | 45 ± 1.4 | 28 | N/A | 40,000 | 8 | 9.9 ± 1.9 |
N/A: Not available.
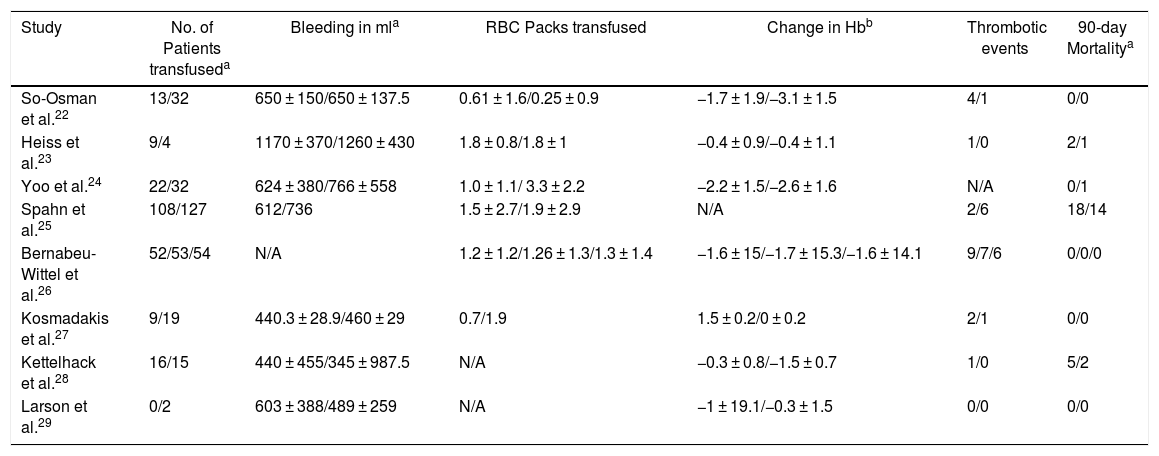
The primary outcomes of this systematic review are the number of patients transfused intraoperatively and postoperatively within 7 days after surgery and thrombotic events, such as complications and the 90-day mortality in the EPO or control group. Secondary outcomes were change in hemoglobin postoperatively, bleeding in milliliters (ml) and the number of average RBC packs transfused per patient (Table 3).
Outcomes of included studies.
| Study | No. of Patients transfuseda | Bleeding in mla | RBC Packs transfused | Change in Hbb | Thrombotic events | 90-day Mortalitya |
|---|---|---|---|---|---|---|
| So-Osman et al.22 | 13/32 | 650 ± 150/650 ± 137.5 | 0.61 ± 1.6/0.25 ± 0.9 | −1.7 ± 1.9/−3.1 ± 1.5 | 4/1 | 0/0 |
| Heiss et al.23 | 9/4 | 1170 ± 370/1260 ± 430 | 1.8 ± 0.8/1.8 ± 1 | −0.4 ± 0.9/−0.4 ± 1.1 | 1/0 | 2/1 |
| Yoo et al.24 | 22/32 | 624 ± 380/766 ± 558 | 1.0 ± 1.1/ 3.3 ± 2.2 | −2.2 ± 1.5/−2.6 ± 1.6 | N/A | 0/1 |
| Spahn et al.25 | 108/127 | 612/736 | 1.5 ± 2.7/1.9 ± 2.9 | N/A | 2/6 | 18/14 |
| Bernabeu-Wittel et al.26 | 52/53/54 | N/A | 1.2 ± 1.2/1.26 ± 1.3/1.3 ± 1.4 | −1.6 ± 15/−1.7 ± 15.3/−1.6 ± 14.1 | 9/7/6 | 0/0/0 |
| Kosmadakis et al.27 | 9/19 | 440.3 ± 28.9/460 ± 29 | 0.7/1.9 | 1.5 ± 0.2/0 ± 0.2 | 2/1 | 0/0 |
| Kettelhack et al.28 | 16/15 | 440 ± 455/345 ± 987.5 | N/A | −0.3 ± 0.8/−1.5 ± 0.7 | 1/0 | 5/2 |
| Larson et al.29 | 0/2 | 603 ± 388/489 ± 259 | N/A | −1 ± 19.1/−0.3 ± 1.5 | 0/0 | 0/0 |
N/A: Not available.
The data analysis was designed by two authors (S.M.E.A. and M.R.). The data were analyzed by authors (S.M.E.A., S.F. and H.G.) using the SPSS, version 23 (IBM Corp, Armonk, New York). Continuous variables were expressed as either mean ± standard deviation or median (minimum-maximum), depending on the distribution of the data. Categorical variables were expressed as numbers and the risk ratio (RR) was used to pool the estimate with a confidence interval of 95% (95%CI) in the forest plots. A 2 × 2 table was drawn up containing patients transfused and not transfused with allogeneic RBC packs in rows, while interventions to determine the risk ratio were shown in columns. The OpenMetaAnalyst Software was used to draw up the forest plots of the outcomes, using the random-effects, generic inverse variance method of DerSimonian and Laird.30 A random-effects model with a confidence interval of 95% (95%CI) was used to pool the RR of the patients transfused with allogeneic RBC packs and the complications and 90-day mortality were reported after interventions, while the standardized mean difference (SMD) was used to pool the estimates for continuous outcomes. The heterogeneity was tested by I2 Statistics. The heterogeneity was considered negligible when there was an I2 of less than 25%, low, when there was an I2 of 26–50%, moderate, when there was an I2 of 51–75% and high, when there was an I2 above 75%.31. The assessment of the significantly moderate or high between-study heterogeneity (I2 > 50%, p < 0.05) for primary outcomes was made by conducting the random-effect meta-regression to forecast the predictors affecting the success and failure of the EPO injection in preoperative anemia. The publication bias will be assessed by the funnel plot and Egger’s and Begg’s tests if 10, or more than 10, studies are found eligible for inclusion.
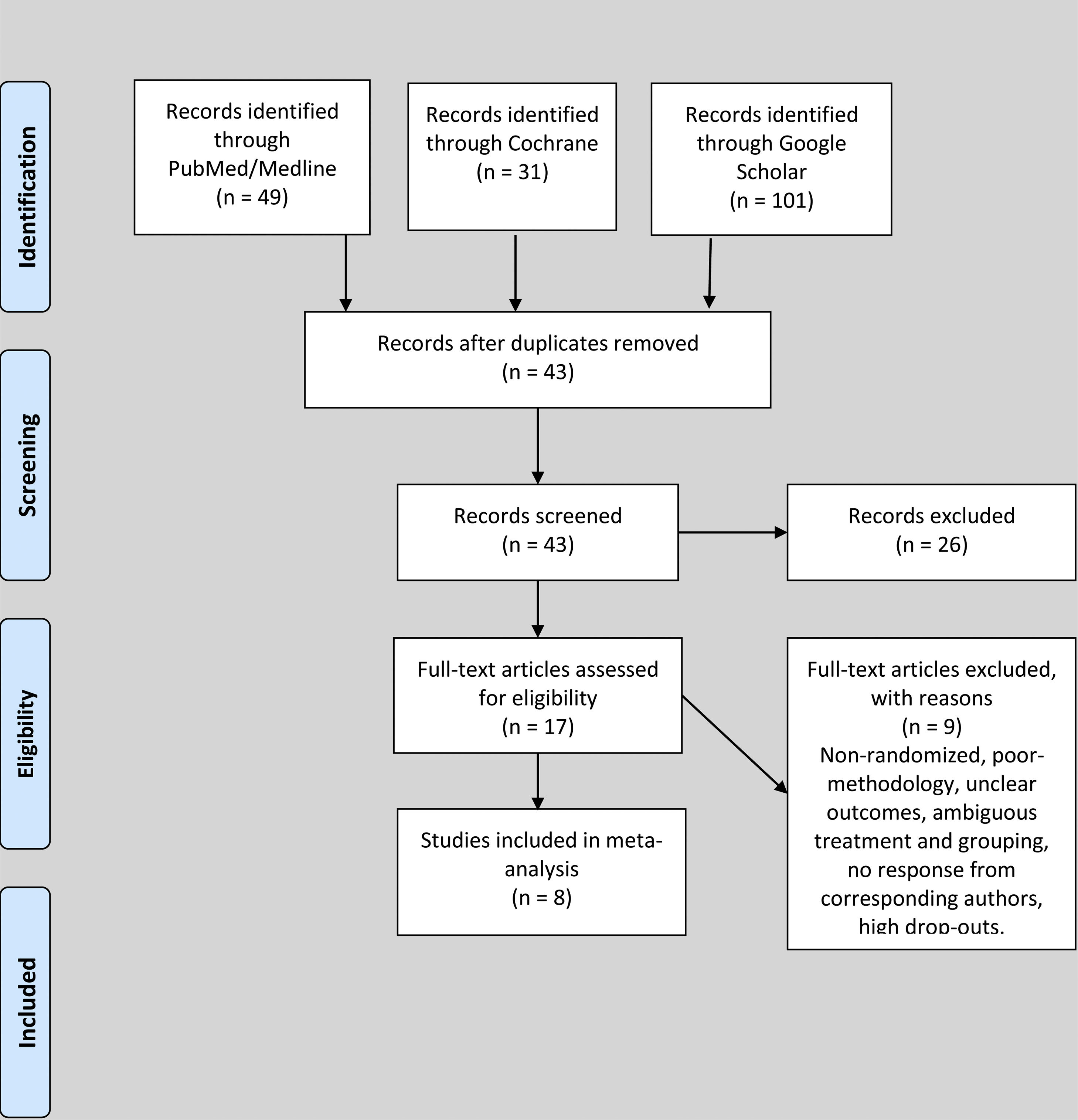
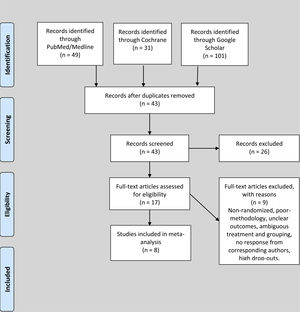
ResultsStudy characteristics and patientsDuring the search for literature from databases, we identified 49 studies from PubMed/Medline, 31 studies from Cochrane and 101 studies from Google Scholar. The studies were screened by titles and 138 duplicate studies were removed. During the abstract screening of 43 articles after duplicate removal, 26 articles were excluded, while full-texts of 17 studies were reviewed for eligibility according to the inclusion and exclusion criteria. Nine studies were excluded after reading the full text due to ineligibility, poor methodology, unclear outcomes, high rate of dropouts and ambiguous grouping. Eight studies, comprising 716 patients with EPO and and 734 controls, respectively, totaling 1,450 subjects, were included in this review, as shown in Figure 1. The studies were based in Netherlands (n = 1), Germany (n = 2), Korea (n = 1), Switzerland (n = 1), Spain (n = 1), Greece (n = 1) and Sweden (n = 1). Four studies were of good quality and four studies were of fair quality. The mean OQSS score was 3.6 ± 1.1. The means of age, day of the first dose, day of last dose, dose in international units (IU), number of EPO injections and preoperative hemoglobin of the candidates in included studies were 65.5 ± 11.1 years, 9.3 ± 10.1 days, 0.7 ± 0.5 days, 86666.7 ± 54650.4 IU, 4.1 ± 2.9 injections and 11.7 ± 1 g/dl, respectively.
Incidence of allogenic transfusionThe primary outcomes of our review considered the allogenic transfusion as a marker of treatment failure. The RR between the EPO group and controls reported by the forest plot is 0.829 (95%CI = 0.688, 0.999, p = 0.049), with a statistically non-significant low heterogeneity (I2 = 48.48%, p = 0.05), as shown in Figure 2. The RR value corresponds to a higher incidence of allogenic transfusion probability in the control group than in the EPO group.
Forest plot showing the relative risk (RR) estimates for the incidence of allogenic transfusion after erythropoietin (EPO) vs. control (Ctrl), in which the boxes show the effect size, with the length of the corresponding line explaining the 95% confidence interval and the diamond-shaped symbol representing the overall effect size.
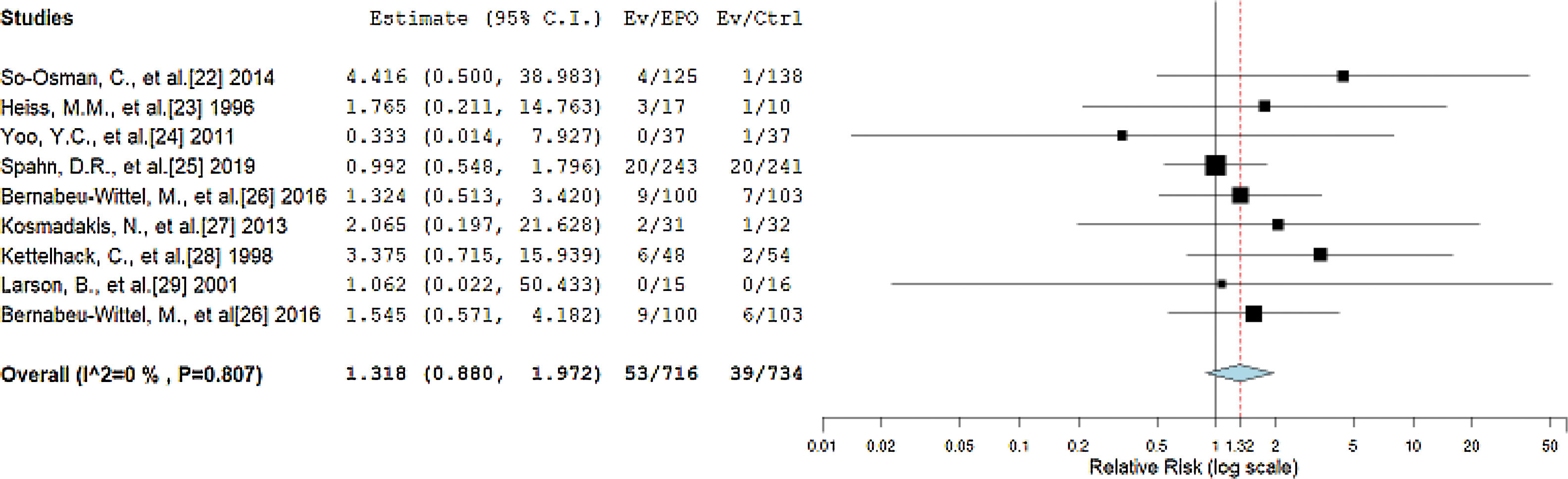
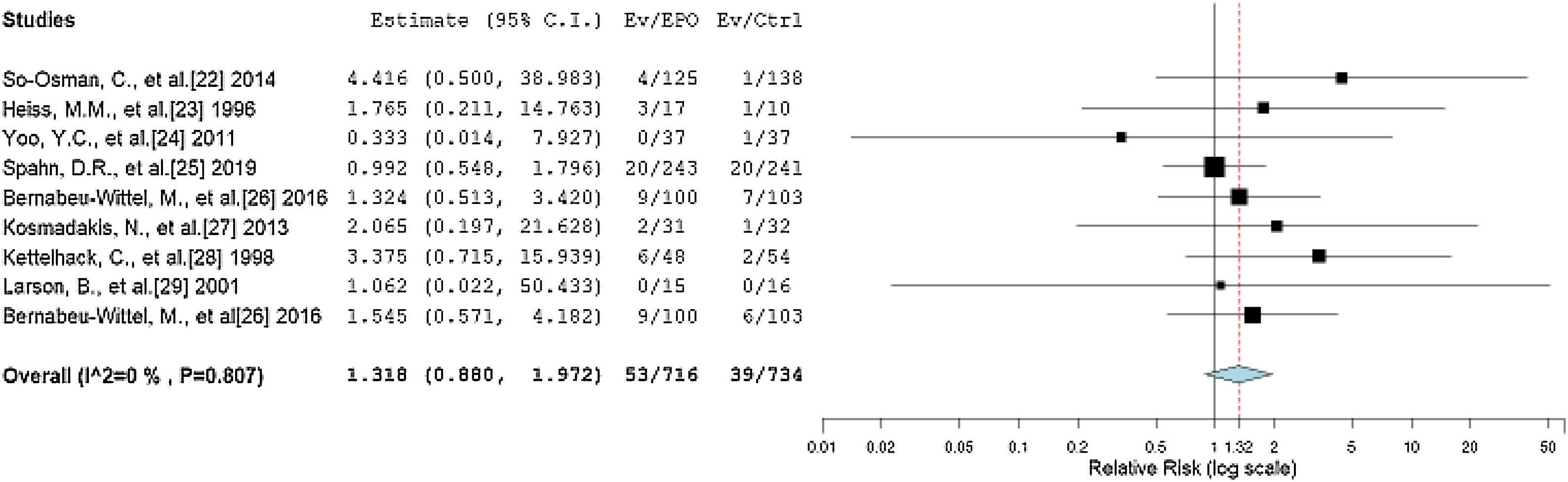
The primary outcomes of our review considered thrombotic events as complications and death within 90 days after surgery as the 90-day mortality after the treatment. The RR between the EPO and control groups reported by the forest plot is 1.318 (95%CI = 0.880, 1.972, p = 0.180), with statistically non-significant negligible heterogeneity (I2 = 0%, p = 0.807), as shown in Figure 3. The RR value corresponds to a statistically non-significant higher incidence of thrombotic events and mortality probability in the EPO group than in the control group.
Forest plot showing the relative risk (RR) estimates for the incidence of complications and mortality after erythropoietin (EPO) vs. control (Ctrl), in which the boxes show the effect size, with the length of the corresponding line explaining the 95% confidence interval and the diamond-shaped symbol representing the overall effect size.
The pooled estimate of the SMD for the change in hemoglobin from the preoperative value to the postoperative value was 0.987 (95%CI = 0.282, 1.693, p < 0.006) with significant heterogeneity (I2 = 95.802, p < 0.001), while for the RBC packs transfused per patient, only five studies reported data quantitatively, in which the pooled estimate by the SMD was -0.181 (95%CI = −0.494, 0.132, p = 0.258), with a significant heterogeneity (I2 = 84.371, p < 0.001). The pooled estimate for blood loss was −0.114 (95%CI = -0.365, 0.137, p = 0.373), with a non-significant low heterogeneity (I2 = 44.249, p = 0.110).
DiscussionThe role of intrinsic erythropoietin has been known for decades and its effect on the bone marrow has previously been described on the molecular level. Recombinant EPO was made commercially available and has been investigated in many trials. Previously written review articles included anemic and non-anemic patients, while in those reviews EPO was shown solely as a blood management strategy, not showing any role of EPO in the correction of the preoperatively anemic patient.32–38 Lin et al.39 and Spahn et al.40 included non-randomized trials with randomized trials, so their studies cannot be regarded as state-of-the-art review articles. Our systematic review included only studies in which the EPO use was investigated to provide optimum hemoglobin levels preoperatively and to decrease the threshold of allogenic transfusions intraoperatively and postoperatively.
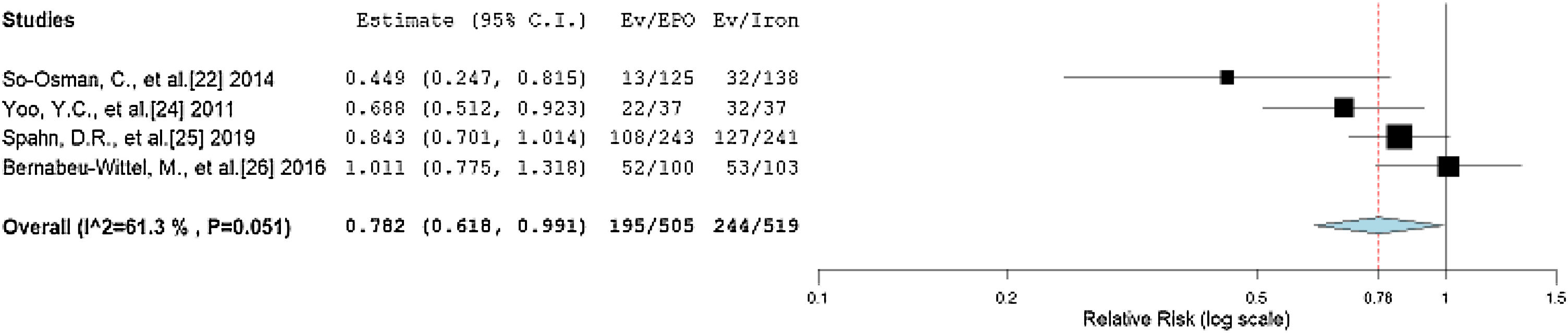
Our forest plot predicted the probability of an allogenic transfusion of 0.829 times in the EPO group, rather than in the non-EPO group. Iron therapy has been used for decades as the preferred option to maintain preoperative hemoglobin levels, so we performed a subgroup analysis to compare the EPO and iron therapies.41–43 We divided the non-EPO group of the trials in this review into an iron therapy group and a placebo group. The iron therapy group received iron doses preoperatively to increase the preoperative hemoglobin, while the placebos received saline or dextrose. A forest plot from the studies using iron therapy compared to those using EPO is shown in Figure 4, with an RR of 0.782 (95%CI = 0.618, 0.991, p = 0.042) and a non-significantly moderate heterogeneity (I2 = 61.304, p = 0.051). The significant relationship shows the necessity for EPO, compared to only iron therapy. However, it is noteworthy that all the studies included in our review have iron therapy prescribed along with EPO. Hence, a combined treatment regimen is superior to the single regimen of iron in the avoidance of intraoperative and postoperative allogeneic transfusions.
Forest plot showing the relative risk (RR) estimates for the incidence of allogenic transfusion after erythropoietin (EPO) vs. iron therapy (Iron), in which the boxes show the effect size, with the length of the corresponding line explaining the 95% confidence interval and the diamonz-shaped symbol representing the overall effect size.
The enhanced effect of EPO with iron can be explained by understanding the physiological aspects of the hormone. The EPO stimulates both the bone marrow stem cells in the production of reticulocytes and the mobilization of the iron reserves.44,45 The increased mobilization of iron due to the hemoglobin synthesis leads to a shortage of iron in the body. Another practical explanation may be given, taking into consideration the global iron deficiency, which leads to a shortage of iron reserves and a decreased response to the EPO therapy alone.46–48 Incorporation of iron with EPO may exacerbate the therapeutic response, producing an earlier and better correction of preoperative anemia.
One of the most feared disadvantages of EPO is the increased risk of thromboembolism that may lead to acute fatal diseases, such as strokes, myocardial infarctions, pulmonary embolisms and bowel ischemias. During our data analysis, we did not find any significant risks of thromboembolism in patients treated with EPO, compared to the non-EPO patients (p = 0.180). Our results showed evidence contrary to that of previously written reviews, which were narrative in nature and lacked proper data analysis.49–52 A theoretical risk of thromboembolic events may be defined by the rise in platelet counts following an EPO intervention. The megakaryocytes as precursors of platelets and erythroblasts as precursors of RBCs share the same myeloid lineage which supports the evidence of the increase in platelets. However, in his review, Beguin 53 reported that thrombocytosis was an immediate effect of high-dose EPO, while long-term use of EPO causes thrombocytopenia. Low to moderate doses of EPO cause mild thrombophilia.
Meier et al.54 in their review described the risks associated with transfusion and anemia. It was the duty of a healthcare worker to consider these risks and decide the optimum management. However, EPO can be utilized in such scenarios to avoid the transfusion-related risks and intraoperative and postoperative risks of preoperative anemia. According to the preoperative blood management guidelines, hemoglobin levels below 10.0 g/dl require blood transfusions. According to our hypothesis, the window hemoglobin concentration between 13.0 g/dl to 10.0 g/dl can be considered for correction by using EPO. The hypothesis is supported by the trials included in our review, as they have a hemoglobin concentration value above 10.0 g/dl, except for Larson et al.29 for whom the average pre-EPO hemoglobin concentration was 9.9 ± 1.9 g/dl.
There were certain limitations that the authors faced in this systematic review. Firstly, the trials included in the systematic review do not include the pediatric population, as most trials focused on the geriatric population. Secondly, only English language articles were included. Thirdly, the grey literature was not searched. Fourthly, publication bias was not assessed due to there being less than 10 studies eligible by inclusion and exclusion criteria for the review.
ConclusionsErythropoietin therapy leads to better outcomes, considering the optimization of preoperative anemia, if given preoperatively in major elective surgical procedures. The benefits of erythropoietin are significantly higher, compared to the control group, while the risks remain equivocal in both groups. The number of trials included in this systematic review is small (n = 8) to conclude that EPO leads to better outcomes, especially in non-anemic patients. However, future randomized trials are necessary to further outline the preoperative days, doses, number of injections and characteristics of patients that are fit for preoperative EPO therapy to provide optimum preoperative hemoglobin in anemic and non-anemic patients.
Conflict of interestThe authors declare no conflicts of interest.