Although there is a vast literature regarding the association between inherited thrombophilia, obstetric complications and the effect of low molecular weight heparin (LMWH), these are controversial and we have not found publications related to additional risk factors other than thrombophilia.
Our objectives were to assess the prevalence of miscarriage, placenta-mediated pregnancy complications and fetal loss in pregnant women with IT, establishing associated risk factors and the effect of LMWH.
Materials and methodsA retrospective cohort of pregnant women with IT was formed. Risk factors considered were: high-risk IT, age ≥35 years, body mass index ≥25 and ≥30, assisted reproductive technology, antiphospholipid antibodies, autoimmune disease, first-degree family history of obstetric complications and personal history of venous or arterial thromboembolic disease, the outcomes being M, FL and PMPC.
Results and conclusionsData from 250 pregnancies in 88 women were obtained.
There were 112 (45%) Ms, 13 (5.2%) FLs and 25 (10%) PMPCs.
High-risk IT was associated with FL (OR=4.96; 95% CI, 1.42–17.3). Antiphospholipid antibodies and family history of obstetric complications were associated with PMPC (OR=7.12; 95% CI, 1.89–26.74, OR=3.88; 95% CI, 1.18–12.78, respectively). The LMWH presented a benefit in the combined outcome (any obstetric complication, OR=0.25; 95% CI, 0.12–0.54) and M (OR=0.41; 95% CI, 0.20–0.82).
We conclude that obstetric complications are common in women with IT. Antiphospholipid antibodies, family history of obstetric complications and high-risk IT might be additional risk factors. The LMWH has an apparent protective effect against obstetric complications, which is consistent with some previous studies.
The management of pregnant women with inherited thrombophilia (IT) is a matter of controversy. Literature is vast regarding the potential association between IT and some obstetric complications. The obstetric complications with which IT has been associated are mainly placenta-mediated complications (PMPCs), followed by miscarriage (M) and fetal loss (FL).1–9
However, results are manifold, and the factors that may predict the presence of obstetric complications in these women are unknown. Different risk factors (RFs) for obstetric complications have been described in the general population, but we have not found reports that assess these factors in women with IT.10–21
We considered the Factor V Leiden mutation (FVL), II20210AG mutation, protein S deficiency, protein C deficiency and antithrombin III deficiency as IT. Nevertheless, considering that Factor XII deficiency, hyperhomocysteinemia and non-FVL-activated non-LAC positive-activated C protein resistance (ACPR) have also been associated with obstetric complications, we have also included them in our study.1,3,22
The effect of low molecular weight heparin (LMWH) therapy during pregnancy in women with a history of obstetric complications has been assessed in multiple studies, with contradictory results. Two systematic reviews with meta-analysis found no significant differences in the rate of live births in patients treated with LMWH, as compared to non-treated patients.23,24 The benefit with LMWH was not established in the subgroup of women with IT.24 However, in the combined results of randomized studies and meta-analyses, the sample is not powerful enough to prove a lack of benefit and establish a recommendation against the use of LMWH in the subgroup of women with IT.
Furthermore, another recently published systematic review found a non-significant decrease in PMPCs in patients treated with LMWH who had experienced previous PMPCs.25 In another systematic review, different results were obtained, and a significant decrease in PMPCs was established.22 Those studies included 42% and 25% women with thrombophilia, respectively. We found three randomized studies that only included women with IT. Two of those studies evidenced that LMWH therapy had significant benefits for women with a history of obstetric complications when started early in the pregnancy.26,27 The third one did not show significant differences. However, in this study, LMWH had been started late in the pregnancy, and the subgroup of women with obstetric complications history was sparse.28
The ALIFE 2 study, which evaluates the efficacy of LMWH in pregnancies of women with IT and a history of obstetric complications, is ongoing.29
The hypothesis of our study is that there are additional risk factors that might impact the bad obstetric outcome in women with IT and that the LMWH therapy might reduce the incidence of obstetric complications in these women.
Our objectives are to assess the prevalence of M, PMPC and FL in the pregnancies of women with IT, establish the associated risk factors that may predispose women to these obstetric complications and evaluate the role of LMWH therapy.
Materials and methodsA retrospective cohort including pregnant women with IT was formed. We included patients with the FVL, II20210AG mutation, protein S deficiency, protein C deficiency, antithrombin III deficiency, factor XII deficiency, ACPR and hyperhomocysteinemia.
Patients were identified through laboratory records and the database of a community hospital's Hematology Department. The enrollment period ran from March 2002 to March 2017. The pregnancies before the IT diagnosis did not receive the LMWH therapy. In all post-diagnosis pregnancies, women were treated before or on the eighth week of gestation.
In the assessment of risk factors, the following ones were considered independent variables: high-risk IT, age ≥35 years, body mass index (BMI) at the beginning of the pregnancy, pregnancy after assisted reproductive technology, presence of antiphospholipid antibodies, autoimmune disease, first-degree family history of obstetric complications and personal history of venous or arterial thromboembolic disease. The high-risk IT was defined as the deficiency of natural inhibitors (protein S deficiency, protein C deficiency or antithrombin III deficiency), the combination of IT or homozygous FVL or the II20210AG mutation. The BMI was used as a categorical variable, considering BMI values ≥25 and ≥30 at the beginning of the pregnancy.
The factor M was defined as a miscarriage before 10 weeks of gestation, the FL was defined as the loss of a pregnancy at 10 weeks or more and the PMPC was defined as preterm birth at less than 34 weeks of gestational age, produced by any of the following: intrauterine growth restriction, with growth <10th percentile, placental abruption, pre-eclampsia, eclampsia or hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome. The STATA, version 12, was used for statistical analysis.
The prognostic impact of the variables considered independent was estimated using the logistic regression model. For the multivariate analysis, variables with a p-value <0.1 were considered. A p-value of <0.05 was considered statistically significant.
To assess the role of the LMWH, we used the M, PMPC and FL, as well as the combination of them, as dependent variables. The analyses were adjusted for the variables that were considered confounders: personal history of venous or arterial thromboembolic disease and antiphospholipid antibodies.
All analyses were adjusted for clustering, considering the fact that some women had more than one pregnancy.
ResultsThe 250 pregnancies of 88 patients were analyzed. The mean age at the time of pregnancy was 33.87 years (SD=5.61, 17–45).
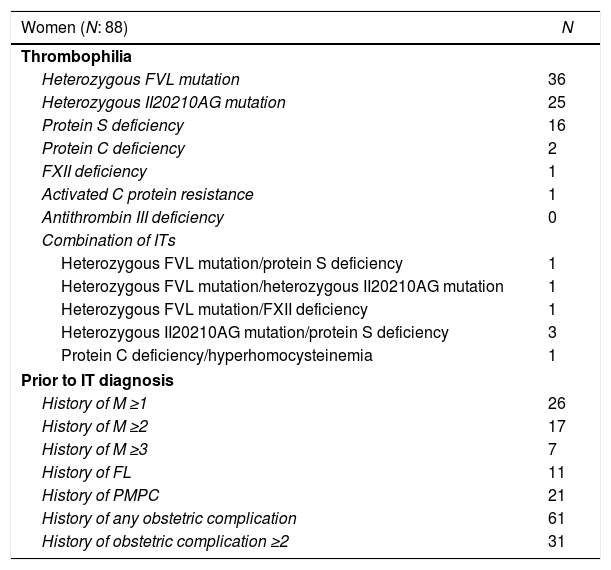
Thirty-six of the 88 women had the heterozygous FVL mutation, 25 had the heterozygous II20210AG mutation, 16 had the protein S deficiency, 2 had the protein C deficiency, 3 had the heterozygous II20210AG mutation/protein S deficiency combination, 1 had the FXII deficiency, 1 had the ACPR, 1 had the heterozygous FVL mutation/heterozygous II20210AG mutation combination, 1 had the heterozygous FVL mutation/FXII deficiency combination, 1 had the heterozygous FVL mutation/protein S deficiency combination and 1 had the protein C deficiency/hyperhomocysteinemia combination. None of the patients had the antithrombin III deficiency (Table 1).
Women's characteristics.
| Women (N: 88) | N |
|---|---|
| Thrombophilia | |
| Heterozygous FVL mutation | 36 |
| Heterozygous II20210AG mutation | 25 |
| Protein S deficiency | 16 |
| Protein C deficiency | 2 |
| FXII deficiency | 1 |
| Activated C protein resistance | 1 |
| Antithrombin III deficiency | 0 |
| Combination of ITs | |
| Heterozygous FVL mutation/protein S deficiency | 1 |
| Heterozygous FVL mutation/heterozygous II20210AG mutation | 1 |
| Heterozygous FVL mutation/FXII deficiency | 1 |
| Heterozygous II20210AG mutation/protein S deficiency | 3 |
| Protein C deficiency/hyperhomocysteinemia | 1 |
| Prior to IT diagnosis | |
| History of M ≥1 | 26 |
| History of M ≥2 | 17 |
| History of M ≥3 | 7 |
| History of FL | 11 |
| History of PMPC | 21 |
| History of any obstetric complication | 61 |
| History of obstetric complication ≥2 | 31 |
Prior to the IT diagnosis, 26/88 women had a history of M, 17/88 had experienced more than one M, 7/88 had experienced more than 2 M, 11/88 had experienced one FL and 21/88 had experienced one PMPC. Most of them (61/88) had experienced one obstetric complication and 31/88 had experienced more than one (Table 1).
The IT was FVL in 38% of the pregnancies (N=95), and the heterozygous II20210AG mutation was present in 32% (N=80), the protein C deficiency, in 2% (N=5), the protein S deficiency, in 16.4% (N=41), the FXII deficiency, in 1.6% (N=4), the ACPR, in 1.2% (N=3) and a combination of 2 ITs were present in 8.8% (N=22). In the last group, 1.6% (N=4) of the patients had the heterozygous FVL mutation/protein S deficiency, 0.8% (N=2) had the heterozygous FVL mutation/FXII deficiency, 1.2% (N=3) had the compound heterozygous FVL/20210Ag mutation, 2.8% (N=7) had the heterozygous II20210AG mutation/protein S deficiency and 2.4% (N=6) had the protein C deficiency/hyperhomocysteinemia. There were 112 (45%) Ms in the 250 pregnancies. This occurred in 35/95 pregnancies in women with the heterozygous FVL mutation, 48/80 pregnancies in patients with the heterozygous 20210Ag mutation, 2/5 with the protein C deficiency alone, 15/41 with the protein S deficiency alone, 3/4 with the FXII deficiency, 1/3 with the ACPR and 8/22 with a combination of 2 ITs.
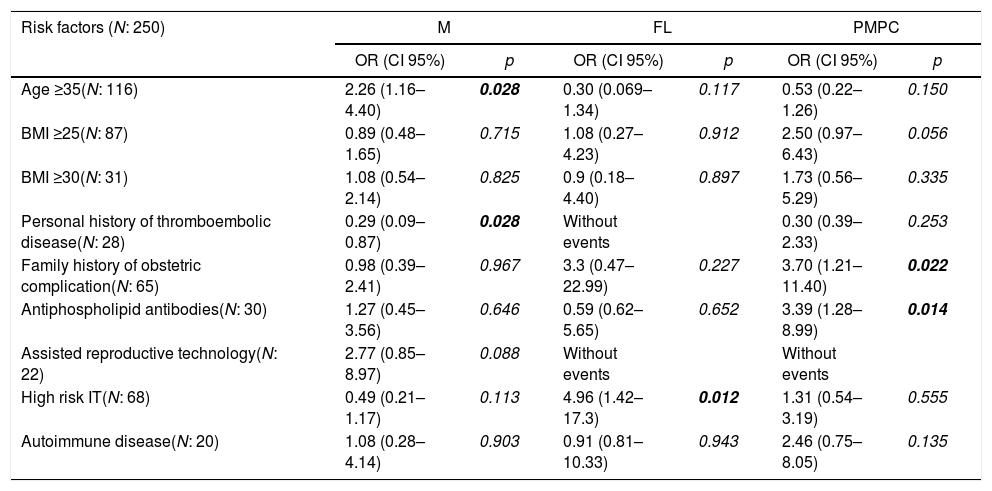
In the univariate analysis, the RFs significantly associated with M were: age ≥35 years (OR=2.26; 95% CI, 1.16–4.40) and assisted reproductive technology (OR=2.77; 95% CI, 0.85–8.97). A factor that was significantly associated as a protector against M was personal history of venous or arterial thromboembolic disease (OR=0.29; 95% CI, 0.11–0.74) (Table 2). The last factor mentioned remained statistically significant when the multivariate analysis was performed (OR=0.32; 95% CI, 0.11–0.93) (Table 3).
Risk factors for M, FL and PMPC. Univariate analysis.
| Risk factors (N: 250) | M | FL | PMPC | |||
|---|---|---|---|---|---|---|
| OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | |
| Age ≥35(N: 116) | 2.26 (1.16–4.40) | 0.028 | 0.30 (0.069–1.34) | 0.117 | 0.53 (0.22–1.26) | 0.150 |
| BMI ≥25(N: 87) | 0.89 (0.48–1.65) | 0.715 | 1.08 (0.27–4.23) | 0.912 | 2.50 (0.97–6.43) | 0.056 |
| BMI ≥30(N: 31) | 1.08 (0.54–2.14) | 0.825 | 0.9 (0.18–4.40) | 0.897 | 1.73 (0.56–5.29) | 0.335 |
| Personal history of thromboembolic disease(N: 28) | 0.29 (0.09–0.87) | 0.028 | Without events | 0.30 (0.39–2.33) | 0.253 | |
| Family history of obstetric complication(N: 65) | 0.98 (0.39–2.41) | 0.967 | 3.3 (0.47–22.99) | 0.227 | 3.70 (1.21–11.40) | 0.022 |
| Antiphospholipid antibodies(N: 30) | 1.27 (0.45– 3.56) | 0.646 | 0.59 (0.62–5.65) | 0.652 | 3.39 (1.28–8.99) | 0.014 |
| Assisted reproductive technology(N: 22) | 2.77 (0.85–8.97) | 0.088 | Without events | Without events | ||
| High risk IT(N: 68) | 0.49 (0.21–1.17) | 0.113 | 4.96 (1.42–17.3) | 0.012 | 1.31 (0.54–3.19) | 0.555 |
| Autoimmune disease(N: 20) | 1.08 (0.28–4.14) | 0.903 | 0.91 (0.81–10.33) | 0.943 | 2.46 (0.75–8.05) | 0.135 |
BOLD values are those with p<0.05 (statistically significant).
Regarding the FL, which occurred in 13/250 pregnancies (5.2%), being 3/95 pregnancies in women with the FVL, 1/80 with the heterozygous 20210Ag mutation, 0 with the protein C deficiency, 7/41 with the protein S deficiency, 0 with the FXII deficiency, 1/3 with the activated C protein resistance and 1/22 with a combination of 2 ITs.
Pregnancies among patients with high-risk IT had significantly more FL, compared with those without high-risk IT (OR=4.96; 95% CI, 1.42–17.3).
Concerning the PMPC, which occurred in 25 pregnancies (10%), 9/95 pregnancies in women with the FVL, 8/80 with the heterozygous 20210Ag mutation, 0 with the protein C deficiency, 6/41 with the protein S deficiency, 0 with the FXII deficiency, 0 with the activated C protein resistance and 2/22 with a combination of 2 ITs.
In the PMPCs, risk factors with a statistically significant value in the univariate analysis were associated with antiphospholipid antibodies (OR=3.39; 95% CI, 1.28–8.99) and the first-degree family history of obstetric complications (OR=3.7; 95% CI, 1.21–11.40) (Table 2). When the multivariate analysis was conducted, these factors remained statistically significant, OR of 7.12 (95% CI, 1.89–26.74) and OR of 3.88 (95% CI 1.18–17.78), respectively (Tables 2 and 4)
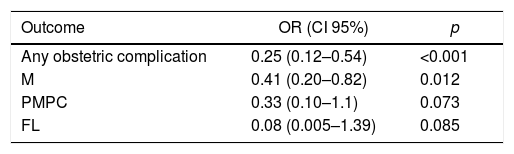
Regarding the LMWH therapy, comparing pregnancies of women who have had not received treatment (pregnancies before diagnosis of thrombophilia), pregnancies with the LMWH therapy had better outcomes. There were fewer Ms, FLs and PMPCs. Nevertheless, it was statistically significant only for M (OR 0.41, 95% CI, 0.20–0.82) and for any combined obstetric complication (OR of 0.25, 95% CI, 0.12–0.54) (Table 5).
Discussion and conclusionOur work evidences a high prevalence of obstetric complications in women with IT. There are other risk factors that might add IT predisposition to different obstetric complications. The LMWH therapy early in the pregnancy might provide significant benefits.
Despite having found a high prevalence of protein S deficiency, all the patients had received their diagnoses at least 90 days prior to conception and the diagnoses were made by measuring the free protein S level using an immunoassay. This high prevalence among our cohort was understandable, as there are two couples of sisters in our cohort.
Literature regarding M describes a prevalence of 15%–25% of M in pregnancies in the general population. The risk found by Wilcox et al. was even higher, around 31%.30,31 After diagnosis of IT, 45% of the pregnancies in women in our cohort had Ms, a prevalence higher than that which had been previously described in the general population.
The prevalence of FL in women with IT was 5.2%. The literature on pregnancy loss is mainly centered upon the recurrent pregnancy loss of early pregnancies or the recurrent or non-recurrent late pregnancy loss. The systematic review with the meta-analysis, published by Rey et al., which included 31 observational studies, established that the FVL implied a higher risk of recurrent pregnancy loss. As for the P20210Ag mutation and protein S deficiency, they were associated with early recurrent pregnancy loss and non-recurrent FLs. The ACPR was associated with early recurrent pregnancy loss.8 Furthermore, the FXII deficiency was assessed in another systematic review by Sotiriadis et al. They found a significant increase in recurrent pregnancy loss in these patients.32
In our study, PMPCs occurred in 10% of the pregnancies in women with ITs.
There is a vast literature regarding PMPCs and IT. The systematic review published by Alfirevic et al. established a significant increase in placental abruption in women with the homozygous or heterozygous FVL mutation, heterozygous P20210Ag mutation, activated C protein resistance and hyperhomocysteinemia. As for pre-eclampsia/eclampsia, it was associated with the heterozygous FVL mutation, heterozygous P20210Ag mutation, protein S deficiency, protein C deficiency and ACPR. Intrauterine growth restriction was associated with the heterozygous P20210Ag mutation and protein S deficiency. There was great heterogeneity among the studies.1
Concerning associated risk factors that may impact the obstetric outcome in women with IT, we only found an observational study where smoking was assessed as an independent variable. A significant increase in pre-eclampsia, intrauterine growth restriction and FL were seen in women with IT who were also smokers.33
In our study, age ≥35 years showed a significant difference as a risk factor for M in the univariate analysis, but not in the multivariate analysis. Overweight and obesity were not a significant RF for any obstetric complication.
The presence of antiphospholipid antibodies was associated with a significant increase in PMPC.
A first-degree family history of obstetric complications was associated in a statistically significant fashion with PMPC. The presence of some high-risk IT was associated with a significant increase in FL.
It is worth mentioning that we observed that previous thromboembolic episodes have an apparent protective effect for both the PMPC and M, which is statistically significant for M. This finding might be explained by a probable difference between women who develop thromboembolic disease and women who have obstetric complications, assuming that women with ITs who were studied due to the presence of thromboembolic disease are less prone to develop obstetric complications than those who were studied due to some obstetric complication.
In relation to the LMWH therapy, our work showed the treatment had a protective effect, both in the combined outcome and in the three obstetric complication groups, with statistically significant results for the prevention of M or for the combined outcome. The results provided in the literature up to now are contradictory. Regarding the outcome of live births, 2 systematic reviews did not find significant differences.23,24 In connection with the PMPC, Rodger el al. published two systematic reviews with meta-analyses. The first one, published in 2014, found benefit in the LMWH therapy,22 whereas the second one found no significant benefit.25 Both primary studies and their results combined in these meta-analyses had little power to prove that using LMWH is not beneficial in the subgroup of women with IT.
In the literature, we have only found three randomized studies that exclusively included women with IT. The first research paper found significant differences in the obstetric outcome in favor of LMWH, as compared to acetylsalicylic acid.26 Subsequently, in 2012, de Vries et al. showed a significant benefit in hypertensive disorders of pregnancy before gestational week 34 when LMWH was added early to the aspirin monotherapy in women with IT and history of obstetric complications.27
Finally, the TIPPS study did not show significant differences in the reduction of venous thromboembolism, M or PMPC in women with IT who had been treated with dalteparin.28 However, this study randomized women in gestational week 12, and the subgroup of women with a history of obstetric complication was too small to draw conclusions in this high-risk subgroup.
Although it is retrospective, our study included mostly women with IT and previous obstetric complication who started the LMWH therapy early. Results were consistent with those of the first two randomized studies mentioned above.26,27
The randomized study ALIFE 2 will provide strong evidence that will probably clarify the controversies that have arisen. That study is ongoing and it evaluates the efficacy of LMWH in pregnant women with IT and histories of obstetric complications.29
On reflection, it is worth mentioning that we have included patients without classic IT.
Coagulation and fibrinolysis balance play an important role in sustaining a normal placental function.34 We did not wish to exclude a prothrombotic factor, such as activated protein C resistance (APCR) or a hypofibrinolytic condition, such as FXII deficiency. Regarding FXII levels, we only order them for patients with prolonged kaolin-activated partial thromboplastin time (KPTT), not otherwise understood.
Despite this shortcoming in our work, the fact that we did include non-classic IT should not impact on the results obtained, as most of the cases with non-classic IT were associated with some classic IT. As an isolated condition, only 1 patient with 4 pregnancies and 1 patient with 3 pregnancies had the FXII deficiency and the APCR, respectively.
We conclude that obstetric complications in women with IT are common, that the presence of antiphospholipid antibodies, a first-degree family history of obstetric complication and high-risk IT are independent risk factors for the development of obstetric complications and that the treatment with LMWH might be beneficial. These findings could offer useful elements for decision-making in the treatment of these women.
However, our results should be taken with caution. Most women are screened after they have been referred to the hematology department due to a previous obstetric complication or thrombotic event. Our data has been collected from a single community-based site cohort, and it has been retrospectively analyzed, with the resulting record bias. Therefore, more studies are required to support our results.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
ContributionsMM Clavijo and CE Casali designed the study and wrote the article; A Ventura, MF Aizpurua and MA Vicente Reparaz worked on database fillings and statistical analysis, and; CV Mahuad and CE Casali revised the intellectual content and interpretation of data. All the authors contributed to the final version to be published.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would to thank Dr. Gonzalo M Garate, Marta E Zerga and Juan Cicco for their contributions in analysis and interpretation of data, and; Sonia Simon and Mariana Fabiano for their assistance in English.