Chronic graft versus host disease (GVHD) is a common complication of allogeneic hematopoietic stem cell transplantation (HSCT) with an incidence of approximately 35–70%.1–3 The risk of acquiring chronic GVHD increases with a history and severity of prior acute GVHD.3 The probability of chronic GVHD increases from 28% in those with no prior history of acute GVHD to 59–85% in those with prior grade II–IV GVHD, according to the National Institutes of Health (NIH) grading criteria.3 Increasing human leukocyte antigen (HLA) disparity between recipient and donor also appears to increase the risk of developing GVHD.3,4 Additional risk factors noted include peripheral blood stem cell (PBSC) transplantation, unrelated donors, female donors (especially if parous) with male recipients, and donor leukocyte infusion.4
While acute GVHD has strong inflammatory components, chronic GVHD has largely been defined by its autoimmune and fibrotic features.1 The leading theory for chronic GVHD implicates damage to the thymus and dysregulation of T helper 2 cells (TH2), which start a cascade that leads to the activation and proliferation of tissue fibroblasts.1 Specific histopathologic criteria based on this understanding and a series of biopsies of different organs dating back to the 1970s exist to help identify chronic GVHD on biopsy.2 The histopathology of chronic GVHD has been described for various affected organ systems, including skin, liver, and gut.2
Muscle tissue is less commonly affected by this disorder and a comprehensive comparison to other inflammatory syndromes in this organ is not yet available.2 The changes found in skeletal muscle can range from mild perimysial lymphocytic infiltrates to extensive endomysial inflammation, with necrosis and regeneration of fibers.2 As the disease process proceeds in stages, the changes associated with chronic GVHD in an organ system can vary.2 Due to this variation, as well as the histopathologic similarity between different causes of myositis, clinical correlation is required for diagnosis.2,5
The symptoms of inflammatory myopathy with chronic GVHD are similar to those found in idiopathic myositis, including muscular pain and proximal muscle weakness.5–7 Creatinine kinase elevations and other laboratory abnormalities seen in muscle breakdown are common, but not always present.5–7 The onset of symptoms is generally seen between 7 and 55 months after transplantation.5 Though treatment with immunosuppressive therapy can be successful, long-term remission remains a challenge.6 This report describes inflammatory myopathy findings in two patients with chronic GVHD.
Case reportPatient 1The first patient is a 66-year-old male with a history of acute myelogenous leukemia (AML), with complex cytogenetics. He received a PBSC transplant from his human leukocyte antigen (HLA)-identical brother, with minor ABO incompatibility after reduced-intensity conditioning with fludarabine and busulfan in June 2011. This was complicated by numerous infections, as well as acute and chronic GVHD, involving the skin and gastrointestinal (GI) tract. His GVHD symptoms were successfully treated with prednisone and budesonide. He experienced a recurrence of his AML in June 2015, when a biopsy of an enlarging right paraspinal mass revealed an extramedullary myeloid sarcoma. He received radiation to the mass, intrathecal methotrexate, and four additional cycles of azacitidine.
In November 2015, he began having symptoms of bilateral lower extremity weakness. Electromyography at that time was consistent with myopathy, with chronic and acute denervation. Repeat thoracic spinal magnetic resonance imaging (MRI) at that time did not reveal a cause of this weakness, with no further enlargement of the paraspinal mass. Lumbar puncture with flow cytometry was negative for leukemia. Budesonide was felt to be contributing to the weakness and was gradually tapered off. He returned in late February 2016 with persistent lower extremity weakness and mild upper extremity symptoms, requiring two weeks of inpatient care. On physical exam, lower extremity strength bilaterally was symmetrically moderately impaired. Creatinine kinase and aldolase were within normal limits. Liver function tests were mildly elevated. The anti-Jo-1 antibody was negative.
Muscle biopsy of left vastus lateralis performed during his hospitalization revealed prominent variation in the size of the few myofibers identified, some with internalized nuclei. Atrophic fibers were seen isolated or in groups, with rare regenerative fibers noted. Histochemical trichrome stain did not reveal the presence of ragged red fibers or rimmed vacuoles. There was increased connective tissue, within the perimysium and endomysium, with presence of abundant adipose tissue surrounding the few surviving fibers. The pathology was consistent with inflammatory myopathy related to chronic GVHD.
The patient was started on prednisone 5mg daily and restarted on budesonide 3mg three times daily. His symptoms persisted for the duration of this treatment schedule, without lasting improvement, following which he was started on four weekly doses of rituximab in May 2016. Though the weakness stabilized with this treatment, it did not resolve entirely. He continued to receive monthly doses of intravenous immunoglobulin therapy (IVIG), but he continued to suffer from repeated infections. He was hospitalized for septic shock in December 2016 and expired from Rhizopus sinusitis in January 2017.
Patient 2Our second patient to develop inflammatory myopathy, consistent with chronic GVHD, was a 65-year-old male with a history of chronic lymphocytic leukemia (CLL), panniculitic T-cell lymphoma, and myelodysplastic syndrome. Remission of CLL, diagnosed in 2005, was achieved with six cycles of fludarabine and rituximab. Subcutaneous panniculitic T-cell lymphoma was successfully treated with cyclosporine in 2011. In 2012, he developed bone marrow failure with evidence of myelodysplastic syndrome (MDS), excess blasts, and fibrosis on biopsy. He was treated with two cycles of azacitidine therapy before therapy was discontinued due to renal toxicity. He received a matched unrelated female donor PBSC transplant in April 2014, following low-dose total body irradiation (TBI) and fludarabine. His post-transplant course was complicated by transaminitis and numerous infections. He developed severe chronic GI and liver GVHD in September 2014 and was started on ursodiol and prednisone 10mg.
In February 2016, he presented with three months of weakness and pain in his lower leg muscles. On exam, he was found to have mild slightly asymmetrical decreases in strength in the lower extremities and no deficits in his upper extremities. Spasticity was also noted but may have been due to a congenital abnormality that had worsened over time. Creatinine kinase and aldolase were within normal limits. Liver function tests were in the high normal range. The anti-Jo-1 antibody was negative.
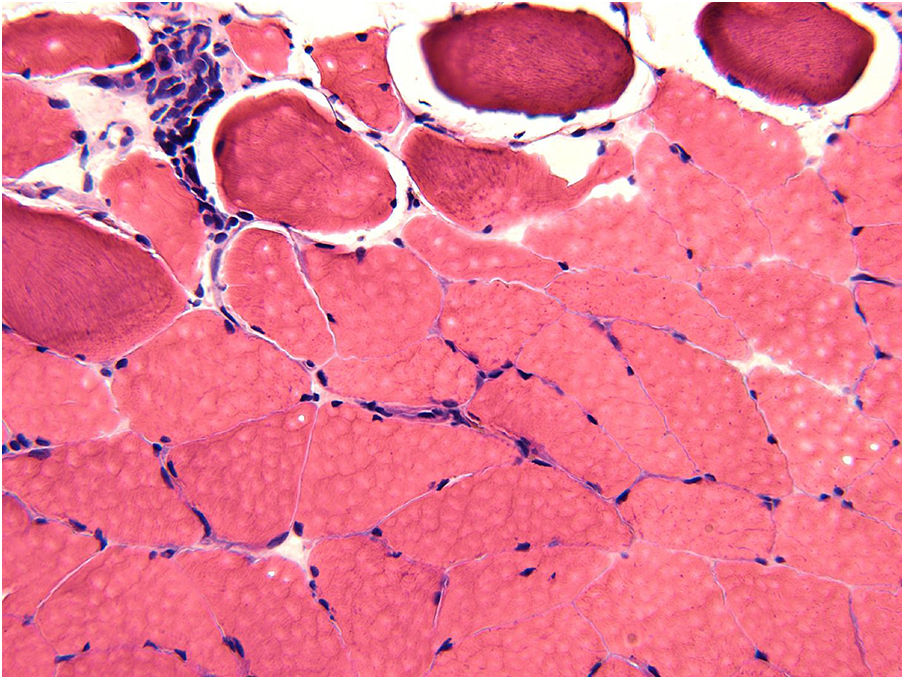
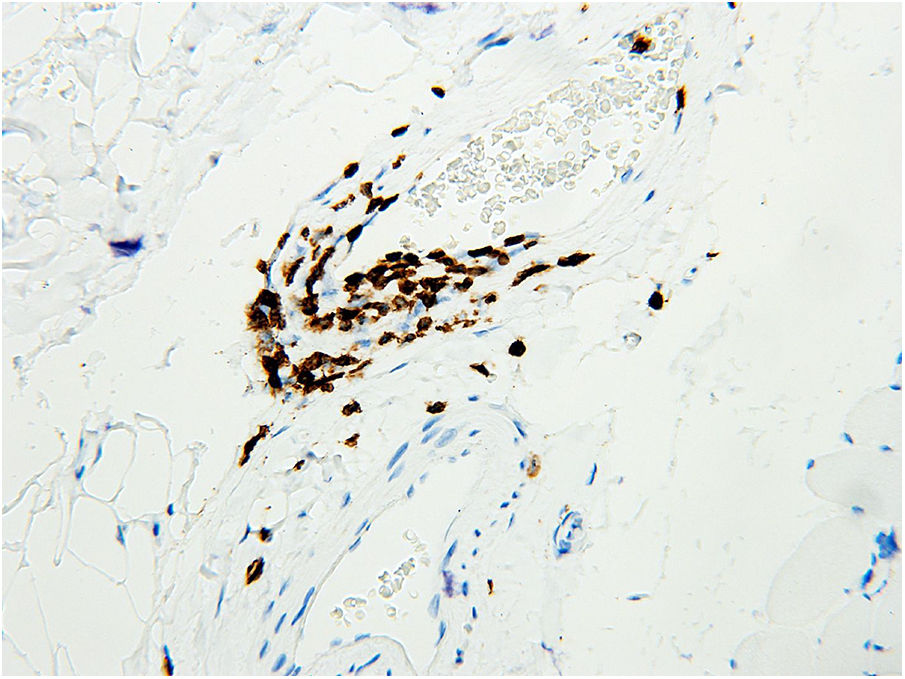
The biopsy of the vastus lateralis muscle was performed in May 2016 and demonstrated small vessel vasculitis, perimysial fibrosis, and scattered atrophic muscle fibers (Figure 1). It showed no evidence of muscle fiber necrosis or inclusion body formation. There was a mixed inflammatory infiltrate of CD3-positive T cells and plasma cells. Immunohistochemical staining for T cells demonstrated lymphocytic infiltration of the perimysial arterial wall (Figure 2). The pathology findings were considered consistent with chronic GVHD-associated inflammatory myopathy. Based on these findings, prednisone was increased to 50mg daily and weekly extracorporeal photopheresis (ECP) treatment was initiated. Monthly treatment with IVIG 40g for chronic GVHD was also started in September 2016, an increase from his 15g dose in June 2016. Due to a lack of symptom improvement, ibrutinib 140mg daily was added in November 2016 and subsequently increased to 320mg daily. The IVIG and ECP treatment was discontinued in December 2016 due to insurance changes for the patient.
By the end of 2017, he had suffered from several falls and was no longer able to ambulate. In January 2018, he was moved to a rehabilitation facility and developed persistent, intermittent infections, bacteremia, dyspnea, and hypoxia. His respiratory function declined to the point of requiring admission to the Intensive Care Unit, after which he was discharged to hospice and passed away soon afterward.
DiscussionInflammatory myopathy is a rare manifestation of chronic graft-versus-host disease. While the pathophysiology is not well understood, the condition is thought to be due to an allo-autoimmune process.5 The diagnosis of inflammatory myopathy includes muscle biopsies with endomysial lymphocytic infiltrate.5 In inflammatory myopathy associated with chronic GVHD, it has been suggested that the donor immune cells invade the native muscle.5 This may be due to abnormal expression of the major histocompatibility complex class I antigen in native muscle fibers.5 Inflammatory myopathy is also associated with specific auto-antibodies and HLA phenotypes, lending further credence to the immunologic basis of the disease.8 Recent studies also discuss pregnancy-derived microchimerism as a potential etiology of the condition.5
Previous reports of chronic GVHD-associated inflammatory myopathy in the literature have been primarily case reports. The clinical presentation often involved limb weakness and fever4,9 and was occasionally associated with cardiac symptoms, such as asymptomatic sinus tachycardia or fatal ventricular arrhythmia.9 The onset followed transplantation and most frequently was subsequent to a steroid taper,8 although it occasionally occurred following donor leukocyte infusion.4 Diagnosis was commonly achieved with a muscle biopsy showing lymphocytic infiltration,5 although elevations in muscle enzymes were not consistent.4,7,9 The treatment included primarily steroids, but also cyclosporine, azathioprine, IVIG, methotrexate, and others.4–6 Rarely were patients reported to have recurrent inflammatory myopathy, but it was nevertheless resolved with further steroid treatment.10 The maintenance therapy most often consisted of prednisone.9
The patients described in this case report also had symptoms of inflammatory myopathy following PBSC transplantation, confirmed with lymphocytic infiltration on muscle biopsy. Neither patient had elevated muscle enzymes or had the anti-Jo1 expression. These patients were not documented to have had cardiac symptoms.
Our patients differ significantly from previously described cases due to their lack of response to treatment. Upon diagnosis of inflammatory myopathy, the first patient was initially treated with prednisone and budesonide, followed by rituximab, followed by IVIG, but his symptoms of muscle weakness were not ever fully resolved. The second patient was treated with increased prednisone, ECP, IVIG, and finally ibrutinib. This patient continued to have symptoms of inflammatory myopathy until his death. Additionally, the increasing doses of immunosuppressant medications required to treat the chronic GVHD and inflammatory myopathy likely predisposed these patients to the infections that ultimately claimed their lives.
Inflammatory myopathy with chronic GVHD is a rare but important disorder in HSCT patients. While most patients respond well to immunosuppressant treatment, the two cases presented in detail here illustrate the difficulty in treating this particular complication with existing regimens.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.