The extracellular matrix protein hyaluronan acid plays an active in role in tumor cell proliferation and invasion. Hyaluronan acid receptors, namely CD168 or the receptor for hyaluronan acid-mediated motility (RHAMM) and CD44 have been implicated in promoting malignancy. There is a lacuna in data on the expression of the receptor in pediatric leukemias.
MethodsPediatric patients with acute leukemia who were diagnosed, treated and followed up in our center were enrolled. The bone marrow biopsies performed prior to treatment were subjected to immunohistochemical staining (54 biopsies: acute lymphoblastic leukemia – 45, acute myeloid leukemia – 9). Blast counts were carried out at diagnosis, end of the induction phase and end of chemotherapy, the minimal residual disease was assessed and follow up details were collected. Positivity was correlated with initial blast count, post-induction blast count, minimal residual disease and patient survival.
ResultsThere was no correlation between the initial blast count and the percentage of blasts with RHAMM expression. The positive correlation between percentage of blasts expressing RHAMM and the post-induction blast count was moderate in acute myeloid leukemia (0.74) and mild in acute lymphoblastic leukemia (0.48). There was a statistically significant difference in RHAMM expression between the two minimal residual disease risk groups (p-value=0.012) with a negative prognostic effect of RHAMM expression. Moreover, a negative prognostic effect of RHAMM expression was noted when patient survival was considered.
ConclusionThis study shows that blasts in acute myeloid leukemia show more RHAMM positivity than those of acute lymphoblastic leukemia indicating the aggressive nature of this type of leukemia. In acute leukemias, patients with high percentages of RHAMM-positive blasts had more post-induction blasts, blasts in minimal residual disease and poorer prognosis.
Acute leukemias are common hematologic malignancies with an incidence of 20–40%.1 Acute lymphoblastic leukemias (ALL) are more common and have a good prognosis in children.2 Though many clinical and hematological factors play a role in the treatment response and prognosis, the role of the extracellular environment in the proliferation of tumor cells affects the prognosis of the patient and is another area requiring further research. An active role is played by the extracellular matrix environment (ECM) in cell proliferation and invasion.3,4 Hyaluronan acid (HA) is a carbohydrate in the ECM which has been found to influence cell proliferation, migration and inhibition of apoptosis in various malignancies.5 The receptor for hyaluronan-mediated motility (RHAMM) and CD44 are HA receptors that have been implicated in promoting malignancy by inducing cell-signaling pathways. RHAMM has not been as well studied as CD44. RHAMM is a soluble protein encoded by the HMMR gene.6 It has variable localization, which can be either on the cell surface, within the cytoplasm, within the nucleus or secreted to the ECM. The cell surface form, occurring as a peripheral or glycophosphatidylinositol (GPI)-linked protein, is designated CD168.7 The cell-surface molecule plays an important role in humoral anti-tumor response.8,9 It alters the cell behavior promoting cell motility during some physiological and malignant processes.10–12 It also plays a key role in hematopoietic stem cell mobilization in normal and malignant processes.13 RHAMM is abnormally expressed in myeloma cells and in other neoplastic B lymphocytes.14 Whether ras influences RHAMM expression in B cell neoplasms or RHAMM alters ras localization to produce a transformed phenotype is not yet clear.15,16 It is upregulated in some tumors, worsening the prognosis. Membrane associated HA-RHAMM interactions regulate cancer cell adhesion in carcinoma of the colon, breast and prostate.17–19 Intracellular RHAMM is associated with cell transformation and tumor progession.20,21 The role of HA in mediating chemoresistance has been expounded but the contribution of RHAMM on this aspect has not been studied well.22,23 RHAMM antibodies have been found to inhibit proliferation, motility and transformation of mesenchymal cells in vitro.24,25 In addition, RHAMM is known to block endothelial cell migration through the basement membrane substrate. One of the most promising leukemia associated antigens in AML and chronic lymphoid leukemia (CLL) is RHAMM (CD168).8,26–30 It has been found that RHAMM can induce strong anti-leukemic immune responses, possibly enabling control of minimal residual disease (MRD).26 However, a recent study performed on AML showed that RHAMM surface/cytoplasmic expression is associated with poor prognosis.31 It is also linked to oncogenesis via ras mutations in murine systems.32 Though some studies have been carried out on RHAMM expression in AML, there is a lacuna in data on the expression of this protein in pediatric leukemias and especially in ALL, which has a good prognosis. This study was put forth as the first of its kind to correlate the prognostic role of RHAMM in acute pediatric leukemias.
MethodsPediatric patients with acute leukemia who were diagnosed, treated and followed up in our center from 2011 to 2015 were enrolled. The Ethics Committee of the institution approved the study. Demographic details were retrieved. Bone marrow aspirates, which were reported as the consensus of three pathologists, were reviewed and the blast count was recorded. The flow cytometry categorization of acute leukemia was taken as the final diagnosis. Bone marrow aspirate was performed at the end of the induction phase of chemotherapy (i.e. 30±5 days) given with curative intention. The bone marrow aspirate was stained with May-Grünwald Giemsa (MGG) and the blast count was done by three pathologists. The blast count at the end of treatment was also carried out in a similar way. The paraffin blocks of bone marrow biopsies (BMBs) done prior to treatment were collected. The paraffin blocks of BMBs which were repeated in a small proportion of patients with relapse were also collected. The BMBs were subjected to RHAMM immunohistochemical (IHC) staining as described below. This included 54 BMBs (ALL – 45, AML – 9) which were done prior to treatment and 11 BMBs that were repeated in a few patients with relapse (≥25% blasts). Six BMBs, including two reactive marrows, two with hemolytic anemia and two with immune thrombocytopenia, were used as negative controls. A tissue biopsy from infiltrating ductal carcinoma of the breast was used as a positive control. The percentage of blasts positive for RHAMM was recorded. MRD assessments by flow cytometry were retrieved and low risk groups were categorized with <0.01%. The patients were followed up from the time of initiating chemotherapy until December 2015. The duration of survival was recorded with the date patients were transferred or lost to follow up being taken as the time of last visit.
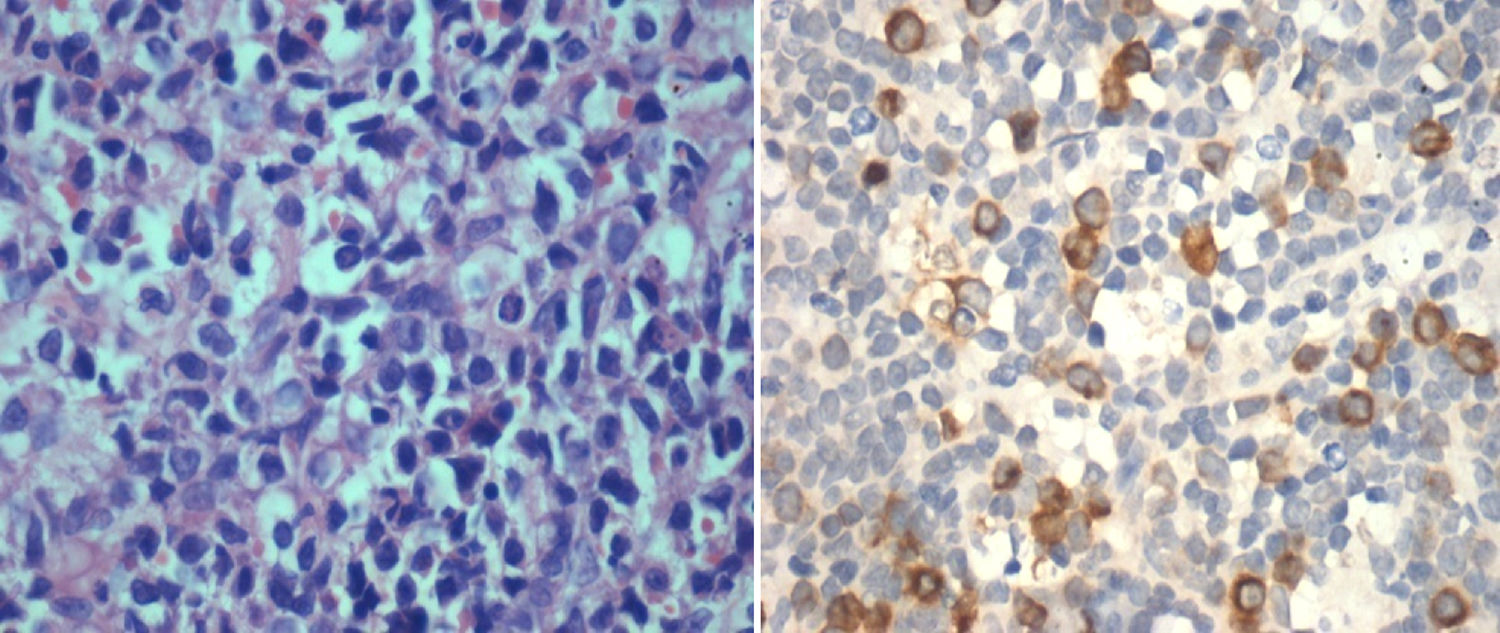
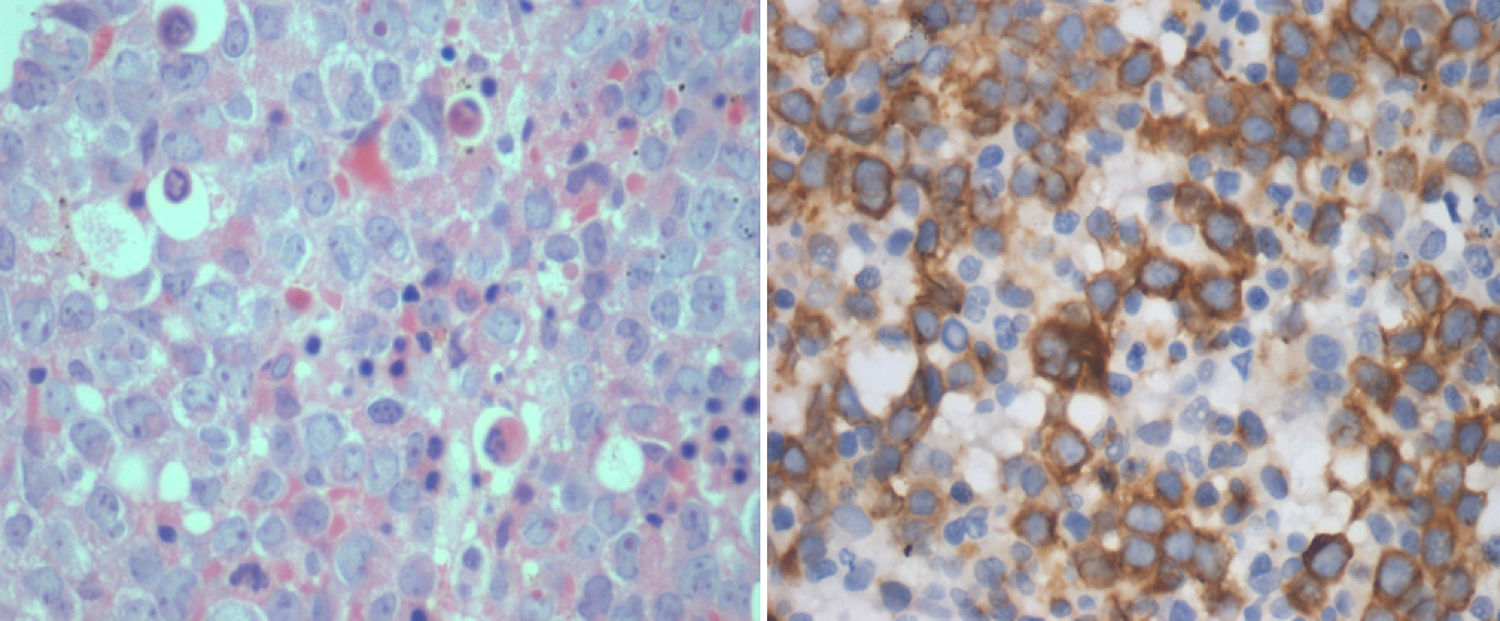
ImmunohistochemistryIHC was performed on paraffin embedded BMB tissue blocks using the BioGenex Xmatrx® staining system with the BioGenex XViz™ Polymer-HRP detection system. Antigen retrieval using a citrate buffer (EZ AR™-1) was performed by boiling the slides at 100°C for 20min. Peroxide block and power block™ reagent was used as a protein blocking reagent. Tissue sections were incubated at 30°C for 60min with the primary antibody [rabbit monoclonal (EPR4055) for CD168, IgG, 1:100, Abcam, USA] and washed twice with a trisodium citrate buffer solution. Super enhancer™ reagent was added for 20min, washed twice with buffer solution, after which poly-horseradish peroxidase reagent was added for 30min at room temperature. Detection was performed using 3,3′-diaminobenzidin (DAB) and counterstained with Hematoxylin and eosin.
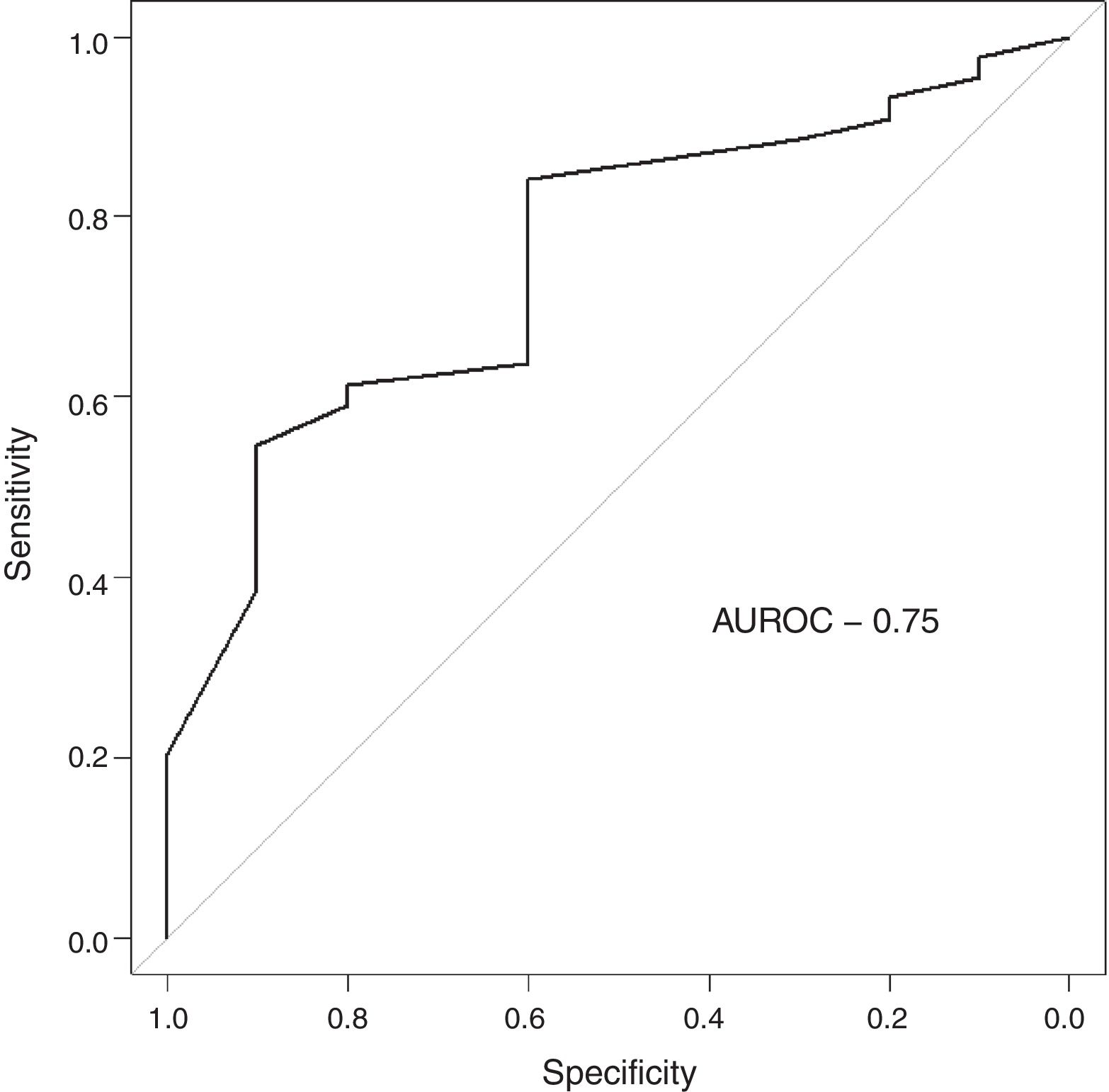
Statistical analysisThe blasts that showed positivity for RHAMM were counted (relative proportion/percentage) by two independent pathologists. Precision was analyzed to be within limits. Statistical analysis was done using GNU PSPPO.8.4 software. Correlations between blast positivity for RHAMM with initial blast count, induction blast count and duration of survival were investigated using the Spearman rank correlation. Receiver operating characteristic (ROC) analysis was used to identify the cutoff of RHAMM to predict survival. A Kaplan Meier survival curve was plotted to compare survival of ALL patients with defined cutoffs of RHAMM obtained through ROC analysis and then statistically compared using the log-rank test. Mann–Whitney U test was used to compare the RHAMM values among high risk and standard risk groups, which were categorized according to the MRD. A p-value of <0.05 was considered statistically significant.
ResultsFifty-four pediatric patients who were diagnosed and treated for acute leukemia at the Sri Ramachandra Medical College Hospital were analyzed. These patients were diagnosed during a span of five years from 2011 to 2016. Among them 45 (83%) had ALL and nine (17%) had AML. The male:female ratio was 1.7:1. Mean ages for ALL and AML were 8 years (range: 2–18 years) and 9.7 years (range: 3–16 years), respectively. The initial blast counts of the bone marrow aspirates ranged from 15 to 98% in ALL and from 26 to 96% in AML. The initial blast count, the RHAMM positivity, post-induction blast count and follow up details are shown in Table 1.
Percentage of blasts at diagnosis and after post-induction chemotherapy (30±5 days), receptor for hyaluronan-mediated motility positivity (RHAMM) and duration of follow up.
| Patients (n) | Initial blast % | RHAMM (CD168)-positive % | Post-induction blast % | Follow up (months) | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | Range | Mean | Range | Mean | |
| ALL (45) | ||||||||
| Alive (29) | 20–98 | 69 | 1–42 | 9 | 0–40 | 3 | 5–48 | 21 |
| Died (7) | 10–98 | 53 | 10–42 | 26 | 0–37 | 2 | 6–40 | 21 |
| Lost follow up (9) | 15–91 | 48 | 0.5–100 | 21 | 0–70 | 10 | 8–52 | 29 |
| Total | 10–98 | 61 | 0.5–100 | 14 | 0–70 | 5 | 6–52 | 23 |
| AML (9) | ||||||||
| Alive (2) | 20–70 | 45 | 10–90 | 50 | 30–65 | 48 | 22–36 | 29 |
| Died (3) | 30–80 | 53 | 40–100 | 70 | 6–86 | 45 | 6–38 | 17 |
| Lost follow up (4) | 26–96 | 55 | 16–70 | 34 | 11–60 | 30 | 12–28 | 22 |
| Total | 20–96 | 52 | 10–100 | 50 | 6–86 | 42 | 6–38 | 21 |
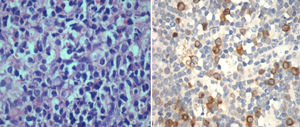
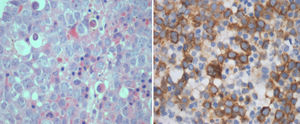
IHC performed with the BMB for RHAMM showed positivity in the range of 1–100% of the blasts (median positivity – 14%) in ALL and 10–100% in AML (median positivity – 50%) (Figures 1 and 2). There was no correlation between the initial blast count at the time of diagnosis and the percentage of blasts that showed RHAMM expression. A moderate positive correlation was found between the percentage of blasts that expressed RHAMM and the number of post-induction blasts in AML (0.74). However, in patients with ALL, though there was a positive correlation between the percentage of RHAMM-positive blasts and the number of post-induction blasts, the correlation was mild and lower than that of AML (0.48). Twenty-one patients with ALL underwent bone marrow aspiration at the end of treatment and the blast percentage varied from 0 to 4 (only morphologically). There was no correlation between RHAMM positivity and the blast count at the end of treatment.
A small population of patients (ALL – 20%; AML – 11%) with relapse underwent further BMBs. The percentage of RHAMM expression in the blasts of the initial BMB and in the biopsy at relapse showed no significant difference. Sixteen patients with ALL had their MRD performed on Day 30 after the initiation of chemotherapy, six (37.5%) of whom were of high risk and ten (62.5%) were of standard risk. The mean RHAMM positivity was 3.7±4% in the patients with standard risk and 27.7±14% in high-risk patients. There was a statistically significant difference in the expression of RHAMM between the two risk groups (p-value=0.012). There was a negative prognostic effect of RHAMM expression when MRD was considered.
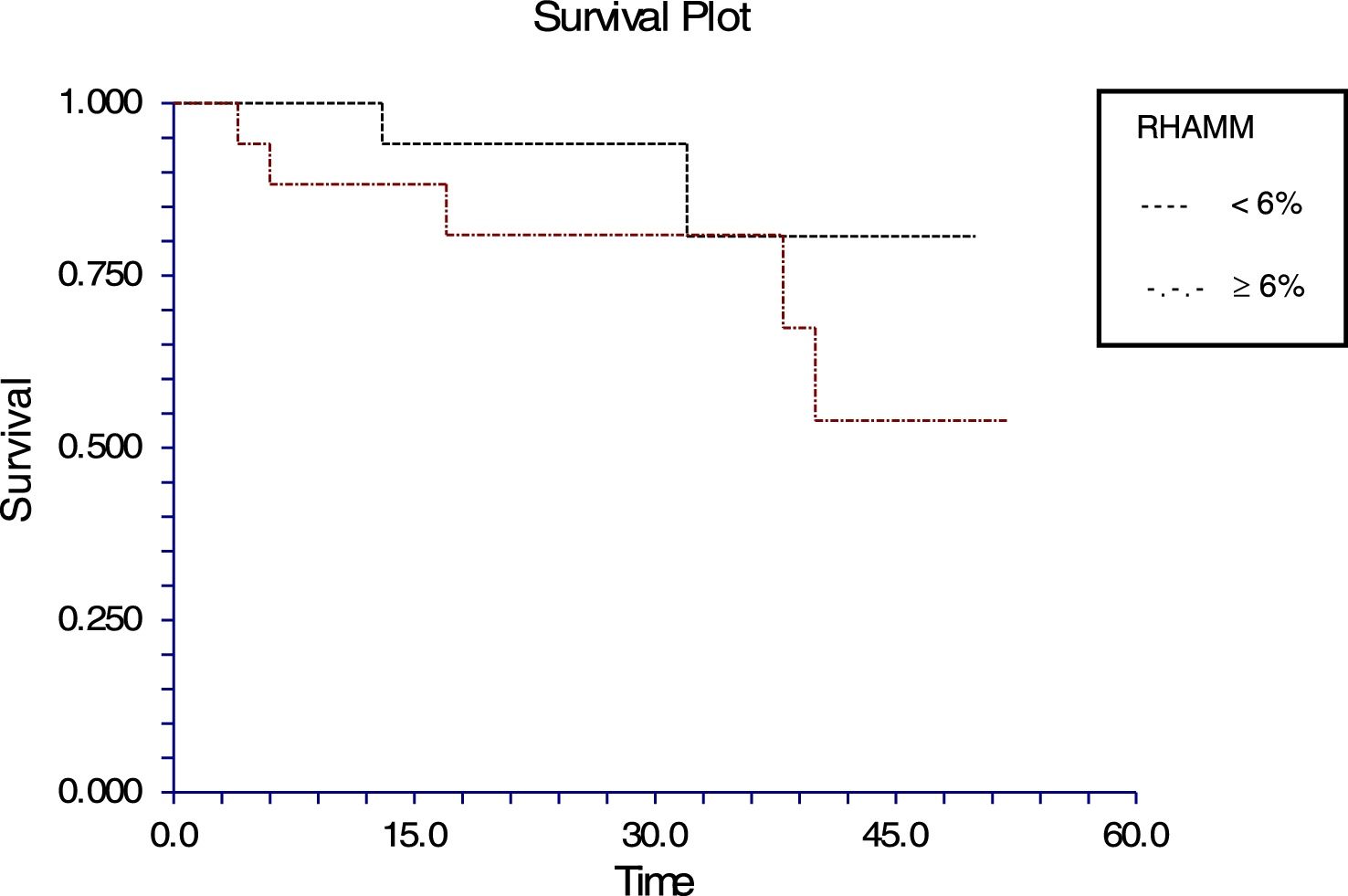
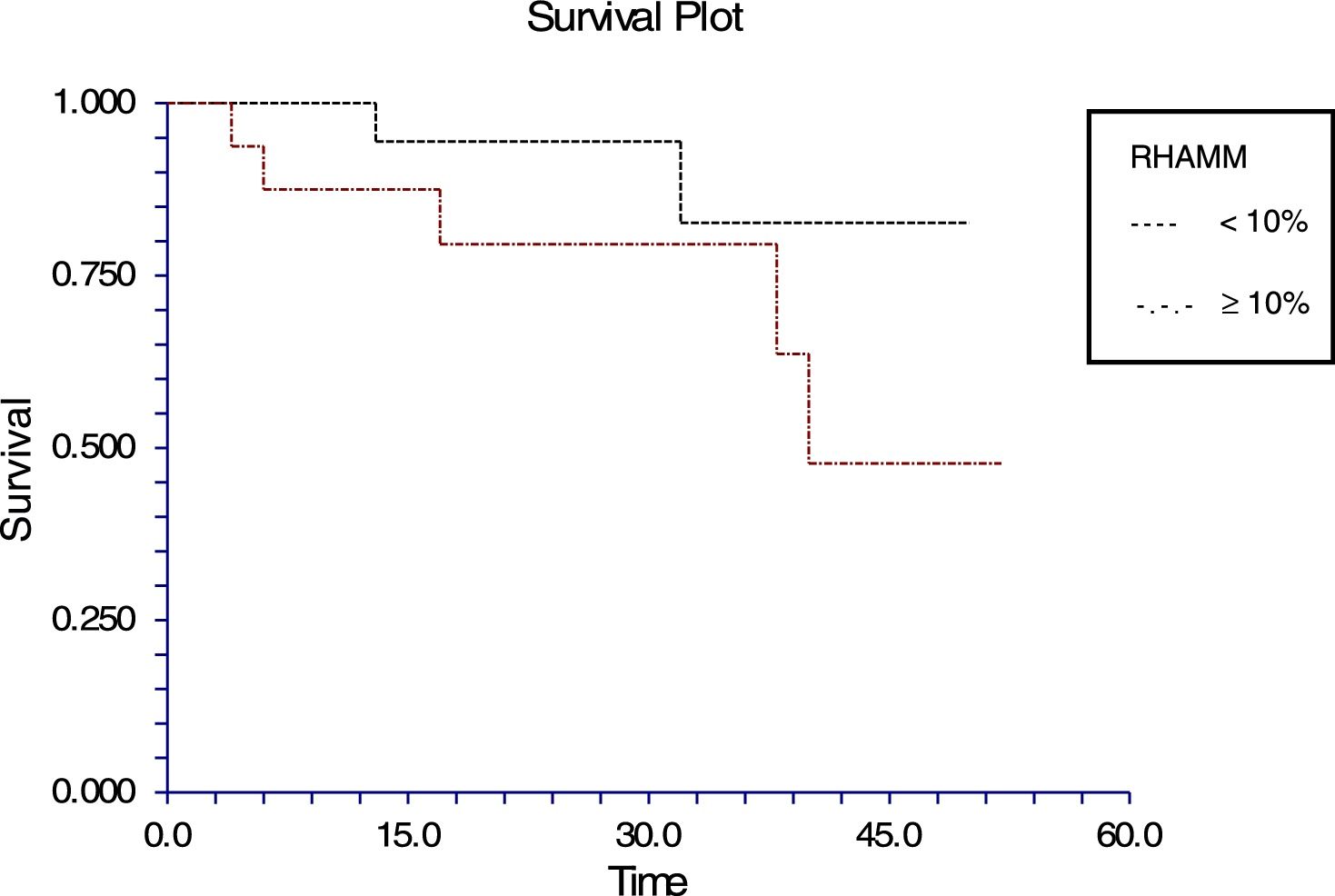
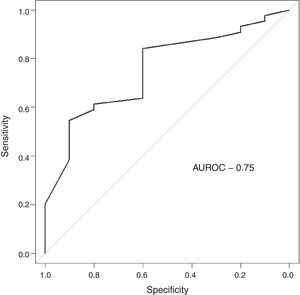
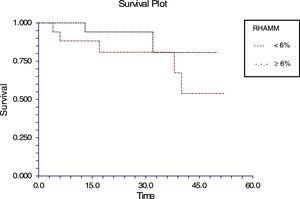
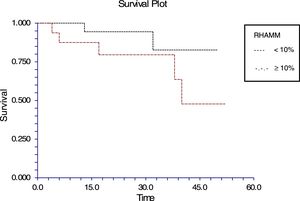
The follow-up of the patients ranged from 6 to 52 months with a mean of 22.5 months in ALL and 22.4 months in AML. During this time, 31 of the 54 patients were alive (ALL – 29; AML – 2); ten patients (ALL – 7; AML – 3) died due to the disease and 13 (ALL – 9; AML – 4) were lost to follow up. No death was associated with chemotherapy toxicity. No patient underwent transplant. ROC curve analysis showed significant discriminatory power for survival (area under the curve=0.75) (Figure 3). This was linked to survival according to the Kaplan Meier method and compared by the log-rank test using the cutoff scores suggested by the ROC analysis. The cutoff scores were >6% blasts (Figure 4) and >10% blasts (Figure 5) for ALL and >30% blasts for AML. The expression of RHAMM in more than 10% blasts showed a significant negative prognostic effect in ALL. However in AML, RHAMM-positive blasts showed a negative prognostic effect when the score was above 30%. The ROC analysis of RHAMM to predict survival demonstrated an area under the curve of 0.75. The cutoff of 6% demonstrated a sensitivity of 80% and a specificity of 60% to predict negative outcomes for ALL patients whereas the sensitivity and specificity were 60% and 61%, respectively if the cutoff was 10%. To predict the negative prognostic effect for AML, patients using a cutoff of 30% gave sensitivity of 60% and specificity of 80%.
The extracellular matrix or peritumor stroma, of which HA is a component, provides tissue homeostasis and is highly essential to regulate malignant cell motility, proliferation, invasion and metastasis. A negative prognostic effect of the high level of HA present in tumor cells and the peritumor stroma had been established in carcinoma of the breast, colorectum and stomach.33,34 It had been postulated that HA transmits signals from the stroma of cells, so that the cellular metabolism is changed.7,35,36 The effects of HA are mediated via their interactions with its receptors of the hyaladherin family namely CD44 and RHAMM.7,22,37–39 Different RHAMM isoforms are available of which cell-surface RHAMM (CD168) interacts with CD44 and is involved in cell motility and signal transduction. Intracellular RHAMM binds to cellular organelles and is involved in tumorogenesis.25,32,40–45 A negative prognostic significance of its overexpression in carcinoma of breast, colorectum, bladder and prostate as well as leukemia and multiple myeloma have been reported by various researchers.14,28,31,34,46–49 It is also said that RHAMM itself is oncogenic when overexpressed and is required for ras mediated transformation.32
In this study of RHAMM expression of the blasts of acute leukemias in children, it was found that the initial blast count at the time of diagnosis had no significant correlation with the percentage of RHAMM-positive blasts, both in AML and ALL. It is possible that other factors apart from RHAMM play a role in the initial blast count. However, it had been found that AML, which has a bad prognosis in childhood leukemias, is associated with more RHAMM-positive blasts than ALL. Whether this contributes to the bad prognosis needs to be studied with more patients. Moreover, the white cell count is considered a prognostic factor rather than the blast count. It has been postulated that redistribution of RHAMM to the cell surface reduces apoptosis and increases the survival of mobilized hematopoietic stem cells.1 Whether this prolonged survival leads to aberrant differentiation toward the leukemic lineage is an area to be studied.
All the patients with ALL except one were precursor B cell type ALL. In this study, the percentage of RHAMM-positive blasts was associated with a mild negative prognostic effect when considering the post-induction blast count. It has been suggested that patients with post-induction blast count >5% (10% of cases) are considered high risk. Only five of the ALL patients in this study were high risk and the RHAMM positivity was higher (29%) than the overall mean RHAMM positivity (14%). However, when correlated with survival, a difference was noted when the RHAMM-positive blast cutoff was 6%; the difference was even more when the cutoff was 10%. The sensitivity and specificity to predict the survival were 80% and 59%, respectively for the cutoff of 6%, while the sensitivity is 60% and specificity is 61% in predicting the survival at a cutoff of 10%. There was a statistically significant difference in the expression between the high and standard risk groups according to the MRD. It had been reported that RHAMM (CD168) was found to be overexpressed in multiple myeloma and B-CLL. This overexpression is essential for ras-mediated transformation.14,32 In a study by Giannopoulous et al. on B cell CLL, it was found that RHAMM (CD168) is a new promising tumor-associated antigen which is expressed on malignant B cells.27,28 The same may hold true for ALL blasts too. Immunotherapy and targeted chemotherapy against RHAMM may be beneficial to a subpopulation of ALL children who express RHAMM in more than 6% of blasts. Furthermore, this may be a promising therapy for those who are at high risk according to their MRD assessment. Since ALL has a good prognosis in children, a small subgroup, who would otherwise relapse with poor outcome, may benefit.
RHAMM (CD168) was identified as one of the leukemia associated antigens.8,26–30 IHC analysis showed a higher percentage of RHAMM-positive blasts in AML than in ALL. There is a moderate negative prognostic effect of RHAMM positivity on the number of post-induction blasts. ROC curve analysis suggested a higher cutoff score of 30% to predict survival. Those children who had more than 30% of RHAMM-positive blasts had a worse survival than those with a lower number of RHAMM-positive blasts. Tzankov et al. used a cutoff of 5% of blasts to predict a poor outcome. However, their study group was adult patients with AML.31 This study included a very small group of patients with AML. In contrast to this study and that of Tzankov et al., Greiner et al. had suggested a favorable prognostic impact of elevated RHAMM mRNA expression in AML.26 RHAMM has a role in leukemogenesis with aggressive AML blasts showing a higher percentage of positivity than those of ALL, which suggests an unfavorable effect of RHAMM expression. It also indicates that targeted therapy against RHAMM may be highly beneficial to children who develop AML.
This study had some limitations. There were very few patients with acute myeloid leukemia and the follow up for survival was variable.
ConclusionThis study is the first study based on IHC staining of BMB to assess the RHAMM-positive blasts in acute pediatric leukemias with correlation with the post-induction blasts and follow up of patients. This study shows that AML blasts show more RHAMM-positivity than ALL blasts indicating the aggressive nature of the leukemic type. In acute leukemias, patients with a high percentage of RHAMM-positive blasts had more post-induction blasts, blasts in MRD and poorer prognosis. Hence, RHAMM (CD168) may be included in the acute leukemia panel for immunophenotyping, so that the parents are counseled accordingly. IHC can also be used as it is a simple, cheap technique. There is a prospective future of targeted therapy against RHAMM in acute leukemias especially in the high-risk category. However, multicentric studies should be performed to assess the therapeutic benefits.
Conflicts of interestThe authors declare no conflicts of interest.
The management of Sri Ramachandra University for the award of GATE project fund and Dr. Suresh Varadarajan, Associate Professor, Department of Community medicine for helping in the statistical analysis.