Burkitt's Lymphoma (BL) is the most common type of Non-Hodgkin's Lymphoma (NHL) in children and can have several types of primary involvement, the most rare being in the bones.1 This manifestation is classified as Primary Bone Lymphoma (PBL) and was studied for the first time in the 20th century by Oberling.2 Currently, it is described by the World Health Organization (WHO) as a malignant lymphoid neoplasm capable of producing one or several masses within bones. It is divided by the WHO into four groups: (1) single tumor, primary site; (2) multiple bone lesions, without visceral involvement; (2) bone lesion with lymph node involvement, and; (4) soft tissue lymphoma with bone involvement.2
The PBLs corresponds to approximately 3–7% of all malignant bone tumors and 5% of extranodal lymphomas, being considered an uncommon disease.2 The disorder results from an infiltration of a mutated lymphocyte in one or more bones, more commonly found in long bones, especially femur and axial skeleton. The most frequent subtype of PBL is the Diffuse Large B-Cell Lymphoma, responsible for about 50% of the cases.3 Among the other possible etiologies for PBL, about 37% are classified as Lymphoblastic Lymphomas, while only 13% of the cases are due to other subtypes, revealing the rarity of BL with bone involvement.3
The present case report aims to discuss the clinical case of a pediatric patient diagnosed with BL with primary involvement of both femurs. In addition, it has the objective of discussing the clinical, radiological, laboratory and histopathological aspects that could assist in the early diagnosis when dealing with this atypical presentation of the neoplasm.
Clinical caseA 9-year-old previously healthy female was referred for evaluation by the oncologist complaining of “pain in both legs for 3 months”. She reported severe pain in both legs, of progressive character, with progression to disability. No other symptoms were described. Due to the high risk for pathological fracture, the patient was instructed to avoid ambulation and therefore used a wheelchair. On physical examination, no significant changes were identified.
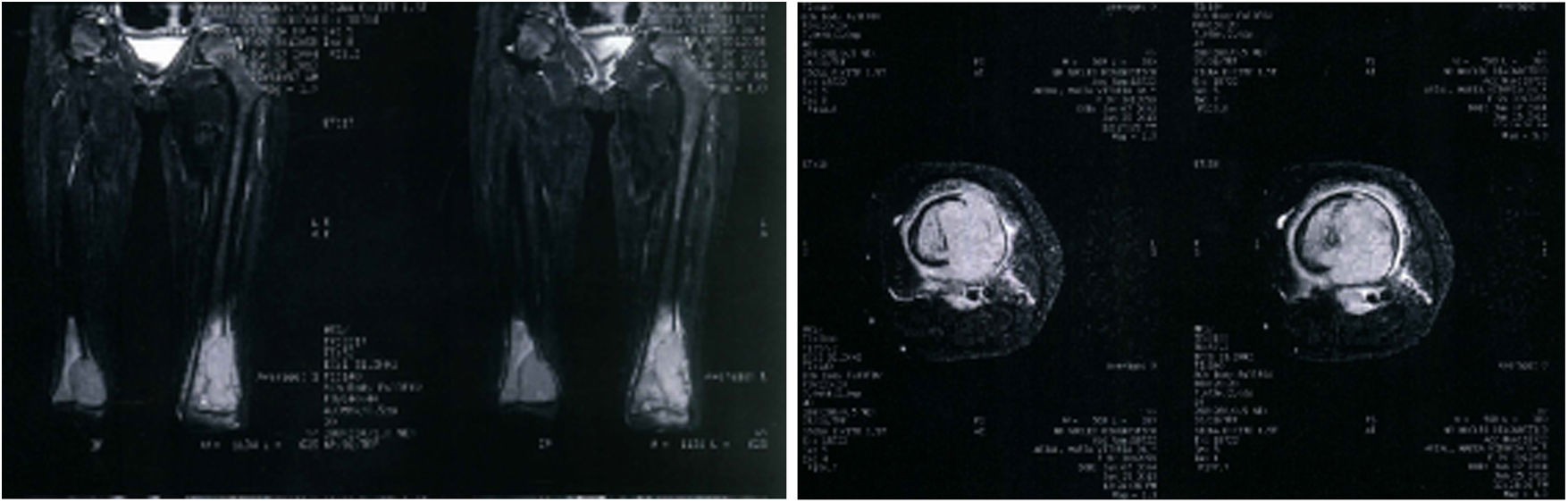
The parents brought a Nuclear Magnetic Resonance Imaging (MRI) of both femurs containing images of coronal and axial sections performed two months prior to this appointment (Figure 1). The description of the MRI included the presence of an aggressive bone lesion in the distal metaphyseal region of the left femur, with a small extension to the epiphyseal region next to the lateral condyle of the femur, associated with an extra-bone extension and subperiosteal component. Lesions with similar characteristics in the medial metaphyseal region of the right femur, with a small epiphyseal extension, were also noted, in addition to cortical destruction and a small extra-bone extension. The lesions were associated with affected lymph nodes in popliteal regions, bilaterally.
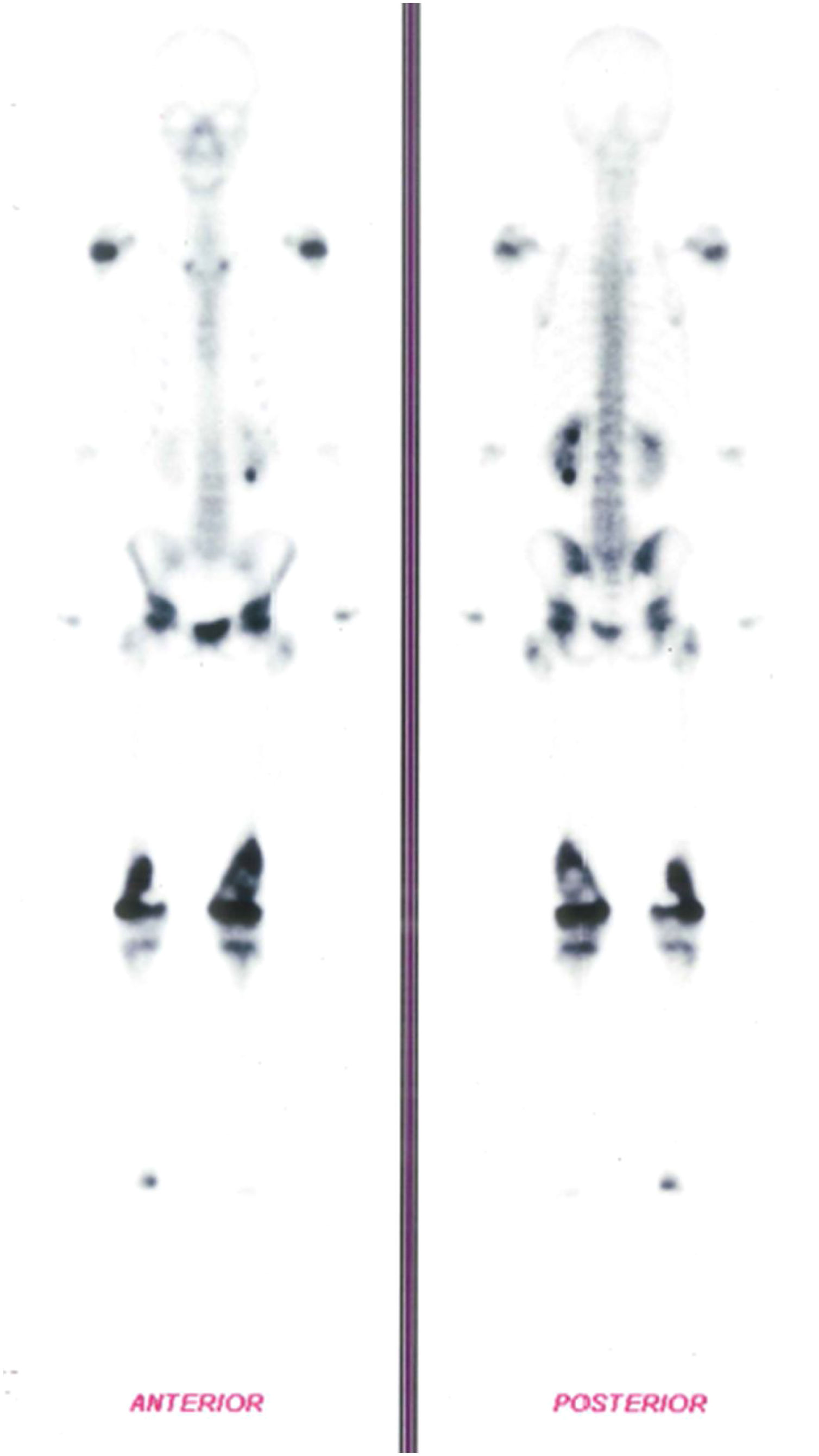
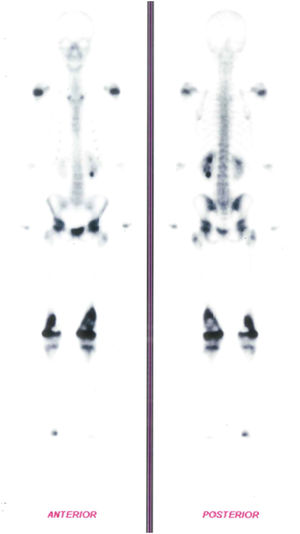
In addition, a Bone Scintigraphy showed signs of osteoblastic activity in the distal third of both femurs, suggestive of bone lesions with a high degree of proliferative activity (Figure 2). Based on the radiological changes that were found, a biopsy of the distal femur had already been performed, with an anatomopathological report of small round and blue cell neoplasms.
Tumor staging was performed with Computed tomography (CT) of the chest and abdomen, immunohistochemical examination, bone marrow karyotype and immunophenotyping performed with bone marrow flow cytometry to search for possible neoplastic foci. The fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) was only available for follow-up, not for diagnosis or staging.
There were no abnormal changes in chest or abdomen in the CT scan. The result of the bone marrow karyotype exam was normal and the immunophenotypic analysis made with bone marrow flow cytometry found no involvement. No significant changes were found in the laboratory tests.
The immunohistochemistry report included “infiltration by high grade B lymphoid neoplasia (CD20 +)”, being BURKITT LYMPHOMA/ACUTE LYMPHOID LEUKEMIA, subtype L3/Burkitt (FAB, WHO 2008) the main diagnosis. The positive markers were CD10, CD20 and CD45RB.
After tumor staging, the patient was then diagnosed with NHL, Subtype Burkitt and classified as belonging to the High-Risk Group. She underwent chemotherapy according to the protocol suggested by the Brazilian Cooperative Group of Lymphomas in Children 2010, Group C.
Currently, the patient is undergoing clinical treatment and has been monitored with annual follow-up at the oncopediatrics service. In addition, the patient currently presents sequels in the coxofemoral region as consequences from the comorbidity and is waiting for orthopedic rehabilitation.
DiscussionFirst described in 1958 by the physician Denis Parsons Burkitt, Burkitt's Lymphoma (BL) consists of a highly aggressive mature B-cell neoplasm that represents about 20–30% of all pediatric lymphomas and 40% of all Non-Hodgkin lymphomas (NHL) in children.1,4
It is more frequent in the pediatric population, with the most affected age group being children between 3 and 8 years of age.5 The disease is more commonly found in the male gender, being in these cases more frequent in the maxilla, mandible and abdomen.5
BL is one of the human neoplasms with the highest proliferation rate, being able to double the number of cells in 24 h.5,6 This neoplasm results from a translation of the MYC gene, one of those responsible for the control of the cell cycle and the apoptosis.4,7 This gene is located in chromosome 8 and its translation occurs more commonly to chromosome 14, resulting in a neoplasm with high rates of proliferation and a high degree of malignancy, but with a good chemotherapeutic response.4,7
The World Health Organization (WHO) subdivides BL into an endemic, sporadic and associated with immunodeficiency form. The endemic subtype is related to regions of Equatorial Africa, Brazil and Pope New Guinea.1 It has an important correlation with previous infections by the Epstein-Barr virus (EBV) and by Plasmodium falciparum, the etiological agent of malaria.1
The sporadic subtype is more common in North America and Western Europe and it is frequent in adults between 30 and 40 years old. It mainly affects the ileocecal region.1 This subtype is also related to the involvement of the central nervous system and bone marrow.7
BLs, classified as being associated with low immunity are frequently related to infection by the human immunodeficiency virus (HIV), may also affect patients with congenital immunodeficiency or those who have recently gone through a transplant.4 The sites most commonly affected in these situations are the lymph nodes, bone marrow and central nervous system.7
Regarding the Primary Bone Lymphoma (PBL), in a study with 18 children with the condition, the estimate of male and female involvement was 1.65:1, respectively, with a mean age of 11.3 years old.3 This information contrasts with the case reported, since the patient is a 9-year-old female.
Considering the signs and symptoms of the condition, the most commonly found are pain, edema and, in some cases, a palpable mass.8 In more severe situations, rupture of the bone cortex and involvement of soft tissues can occur.9 Pathological fractures are not uncommon and in these cases lead to secondary complications.
The diagnosis involves researching the region through imaging tests associated with the analysis of biopsies of the suspected mass. In addition, it is necessary to perform histopathological and immunohistochemical research for a definitive diagnosis.3 In conjucntion, these tests allow for the tumor staging and evaluation of the indicated treatment. In cases of a high degree of suspicion and radiography without alterations, it is important to request a contrasted MRI that can accurately identify changes in the bone marrow.3
The association of imaging tests helps to investigate the involvement of neighboring and distant structures. The tests that can be used are X-Ray, Computed tomography (CT), Bone Scintigraphy and PET Scan. In addition, a CT scan of the head and neck, abdomen and pelvis are necessary for staging.7
Local radiographic findings are variable, going from an apparently normal bone to a lytic or sclerotic lesion, or characteristics of an infiltrative process, being the destructive lytic pattern the most common. In addition, the “moth-eaten” pattern is described as characteristic of bone involvement.9
In association, the FDG-PET/CT has become one of the exams of choice for providing better accuracy, both for the initial diagnosis and evaluation. Moreover, it can also be used for subsequent follow-up, analysis of the therapeutic response and the identification of possibly malignant changes in early stages.6 Unfortunately, in the present case the PET/CT was not available for diagnosis and initial staging, so it is possible that there were other injuries undetected by the available methods used.
The definitive diagnosis of BL depends on the investigation of the cellular origin of the affected site by histopathological analysis, performed from the local biopsy.6 The findings usually demonstrate abnormal tissue architecture with the presence of atypical monomorphic lymphocytes, with rounded nuclei and basophilic cytoplasm.7 These are generally surrounded by macrophages in the process of apoptosis, classically described as a “starry sky” pattern.7,4
The immunohistochemical examination consists of researching the cellular markers expressed in the BL cells and this enables the etiologic diagnosis and identification of the tumor malignancy. In this case, it can be positive for Surface immunoglobulin IgM (sIg), CD5, CD10, CD19, CD20, CD22, BCL6, CD38, CD43, CD77, CD79a and HLA-DR and it will be negative for CD5, BLC-2, TdT and CD23.4,6,7
In addition, bone marrow puncture is indicated for further analysis of local involvement, called “Burkitt's Leukemia”, one of the presentations of BL defined by the presence of more than 5% of malignant cells in a spinal cord aspirate.1,7
In situations with bone involvement, the diagnosis of PBL is not made quickly because there are a large number of differential diagnoses, including other neoplasms and rheumatological or infectious diseases.2 Other primary bone tumors can also be classified as possible differential diagnoses, including Osteosarcoma and Ewing's Sarcoma.2
Conflicts of interestThe authors declare no conflicts of interest.