To determine the frequency of folic acid deficiency in consecutive serum folate determinations and to determine whether there was a significant decrease in serum folate deficiency after folate was added to wheat flour.
MethodsA retrospective descriptive observational study was performed of consecutive folate measurements at the Hospital Privado Universitario, Cordoba, Argentina.
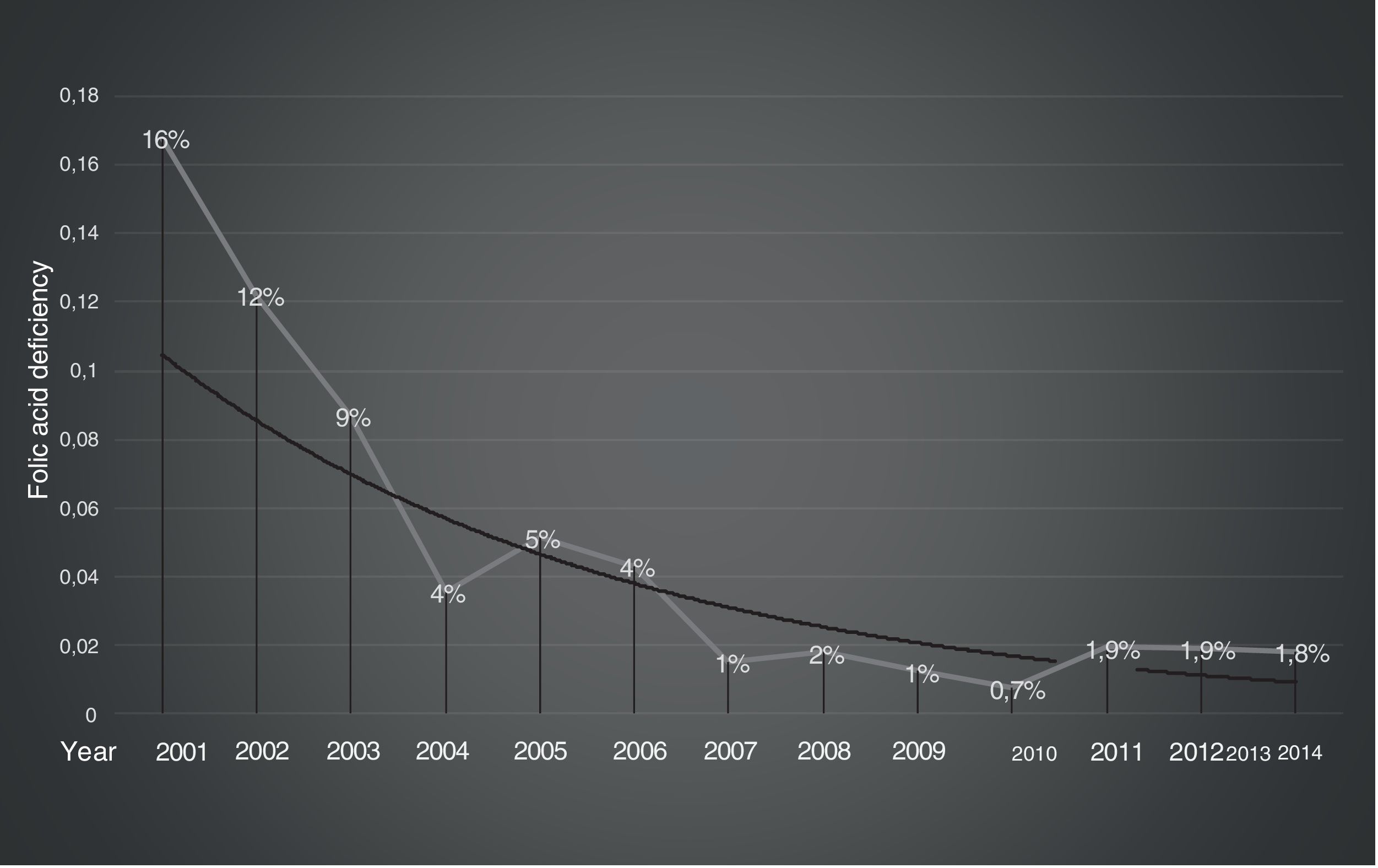
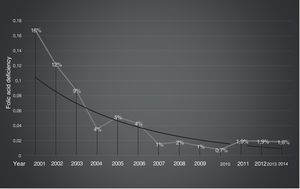
ResultsTwo cohorts were analyzed: 1197 folate measurements between 2001 and 2008 (before supplementation) and 3335 folate measurements from 2009 to 2014 (after supplementation). Folate deficiency was found in 84/1197 (7%) subjects in the pre-supplementation group and in 58/3335 (1.73%) after supplementation. The prevalence of folate deficiency was 12% between 2001 and 2003 when folate was not added to flour compared to 4% in 2004–2007 (p-value<0.0001) when folate was added to the flour but no widespread use was documented.
ConclusionsIn the studied population, the prevalence of serum folic acid deficiency after folate supplementation was low at 1.73%. There was a significant decrease in folate deficiency after folate was added to wheat flour. Given the low prevalence of folic acid deficiency observed in this and similar studies, and the observed change with supplementation, we conclude that routine measurement of serum folate is of limited clinical use.
Folic acid is a water-soluble vitamin in the vitamin B complex family. Its active derivative, 5,6,7,8-tetrahydrofolic acid, usually called tetrahydrofolate, is present naturally in foods such as leaf vegetables, legumes, egg yolks, liver and some citrus fruits.1,2 Folic acid is essential for normal cell growth and multiplication, but the bioavailability of natural folate is lower than that of folic acid, a synthetic compound used in supplements and fortified foods. Deficiencies in folate and vitamin B12 have been recognized as some of the causes of macrocytic anemia. For this reason, folate has been prescribed to patients with hematological alterations.3
Neural tube defects are one of the most common congenital malformations of the central nervous system, ranking second in frequency after heart defects.4,5 The relationship between neural tube defects and folic acid deficiency was suggested more than 50 years ago. Since then, it has been recognized in numerous clinical and experimental studies.6 Folic acid supplementation during the periconceptional period prevents a substantial proportion of these malformations. In addition, neuropsychiatric signs associated with folate deficiency, such as sleep and memory disorders, irritability and seizures, have been observed.7 In some cases, this deficiency has been associated with peripheral neuropathy, cerebellar syndrome, depression, and dementia.8,9
The daily requirement of folate for a human adult is 50–100μg, with these requirements increasing to up to 400μg during pregnancy. Different countries on the American continents, including Brazil, Canada, Costa Rica, Chile, El Salvador, the USA, Guatemala, Honduras, Mexico, Nicaragua, Panama, and Peru, have implemented mandatory folic acid fortification of commonly consumed foods, generally flour, as the main prevention measure. In Europe, voluntary fortification has been chosen.10–12 In Argentina, the enrichment of wheat flour with iron, folic acid, thiamine, riboflavin, and niacin was established by Law No. 25,630, which was enacted in July 2002. The regulations (Decree No. 597/03) were published in the Official Gazette on August 14, 2003, and the population began to receive these dietary supplements in 2004.
The amount of folic acid added (2.2mg/kg of flour) was based on an estimated consumption of 160g/day of bread (country's average per adult), which predicts an additional consumption of folate of around 250 micrograms per day. The objective of Law 25.630 was the prevention of anemia and malformations of the neural tube. It has been shown that the current intake of folate in a representative sample of the population of women of childbearing age was adequate, and that the fortification of wheat flour contributes more than 40% toward this intake.11 In addition, a significant reduction in the prevalence of neural tube defects and related mortality has been shown.13,14 In the same population, the prevalence of inadequate serum folate levels was low.15
The purpose of this work was to determine the frequency of folic acid deficiency in consecutive serum folate measurements performed at the Hospital Privado, Universitario de Córdoba in Argentina from 2001 to 2008 (pre-supplementation) and from July 2009 to June 2014 (post-supplementation). Additionally, the prevalence of folate deficiency was analyzed according to the age group, sex, and risk factors in the post-supplementation cohort from 2009 to 2014. The medical conduct adopted by the treating physicians when detecting folate deficiency was also evaluated.
MethodsA retrospective descriptive observational study was performed of consecutive serum folate measurements at the Hospital Privado, Universitario Centro Medico de Córdoba, from January 1, 2001 through December 31, 2008 and from July 1, 2009 through June 30, 2014. Our hospital serves a population of mostly middle- to high-income individuals. The first group (pre-supplementation) was comprised of 1197 patients and the second cohort of patients (post-supplementation) of 3335. Duplicate measurements were discarded.
Until 2010, the method used to measure serum folate was electrochemiluminescence (Cobas®; Roche). Subsequently, serum folate was measured using chemiluminescence (Architect Integrated System®; Abbott Diagnostics).
Deficiency was defined as a serum folate level ≤3ng/mL, a risk of deficiency between 3.1ng/mL and 6ng/mL, and a normal range≥6ng/mL, according to the recommendations of the National Health and Nutrition Survey 2007 (Plan Federal de Salud) of the Ministry of Health of Argentina.15 The prevalences of serum folate deficiency over the years were analyzed for both groups. Based on the electronic medical records, the age and sex were investigated in the post-folate supplementation group. Within the deficiency group, information was gathered about the reason to request a test of folate levels, the comorbidities, mean corpuscular volume (MCV) associated with the measurement and the medical conduct adopted. The normal reference range for MCV is 80–96fl in adults.16
The variables are expressed as means, medians, and percentages. The proportions were compared using a chi-squared test and a p-value<0.05 was considered significant. Statistical analyses were performed using the Arcus Quickstat Biomedical 1.1 program. The different graphs were created using the IBM Statistical Package for the Social Sciences (SPSS) version 21.0.
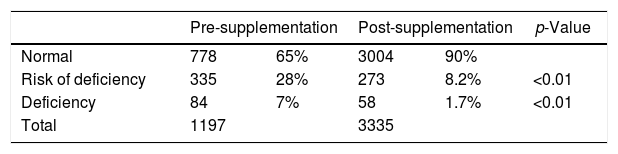
ResultsTwo cohorts of patients were analyzed. In the first group (pre-supplementation), 1197 measurements were recorded with 84 (7%) cases having folate deficiency, 335 (28%) were at risk of a deficiency, and 778 (65%) had normal results. In the second group (post-supplementation), of 3335 measurements, 58 (1.7%) cases had folate deficiency, 273 (8.2%) were at risk of a deficiency, and 3004 (90%) had normal results (Table 1).
The prevalence of deficiency per year was significantly higher before supplementation than after supplementation (7% vs. 1.7%; p-value<0.0001). Furthermore, the prevalence of 12% in the period without wheat flour supplementation (2001–2003) began to decline to 4% in the period from 2004 to 2007 (p-value<0.0001). The serum folate levels reached stable values by the third year after starting supplementation (2007) and they remained stable at around 2% during the seven years thereafter (Figure 1).
After supplementation, the deficiency in folic acid per age group was greater between the ages of 50 and 74 years old (2.2%) and in over 74-year olds (2.3%).
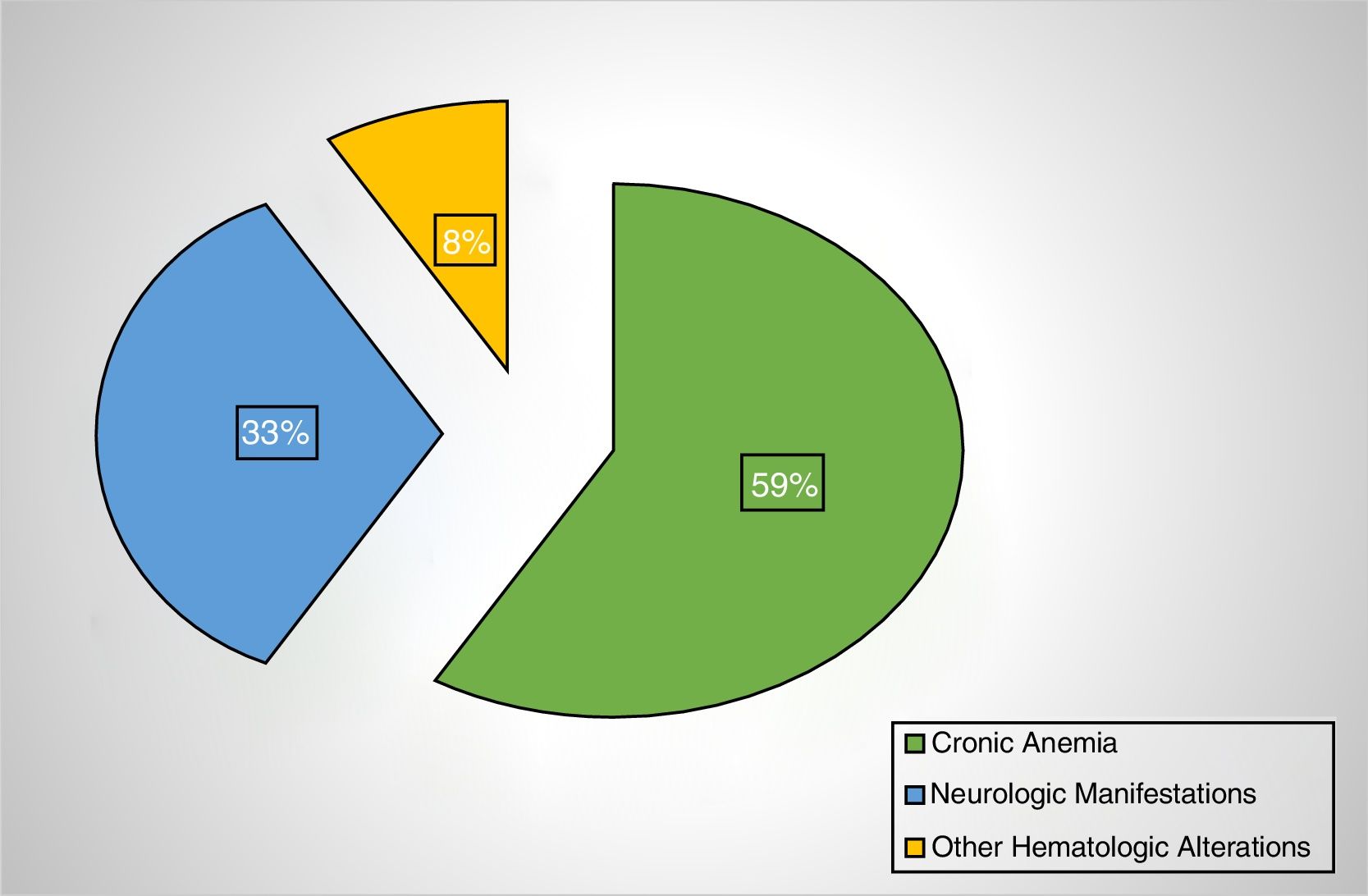
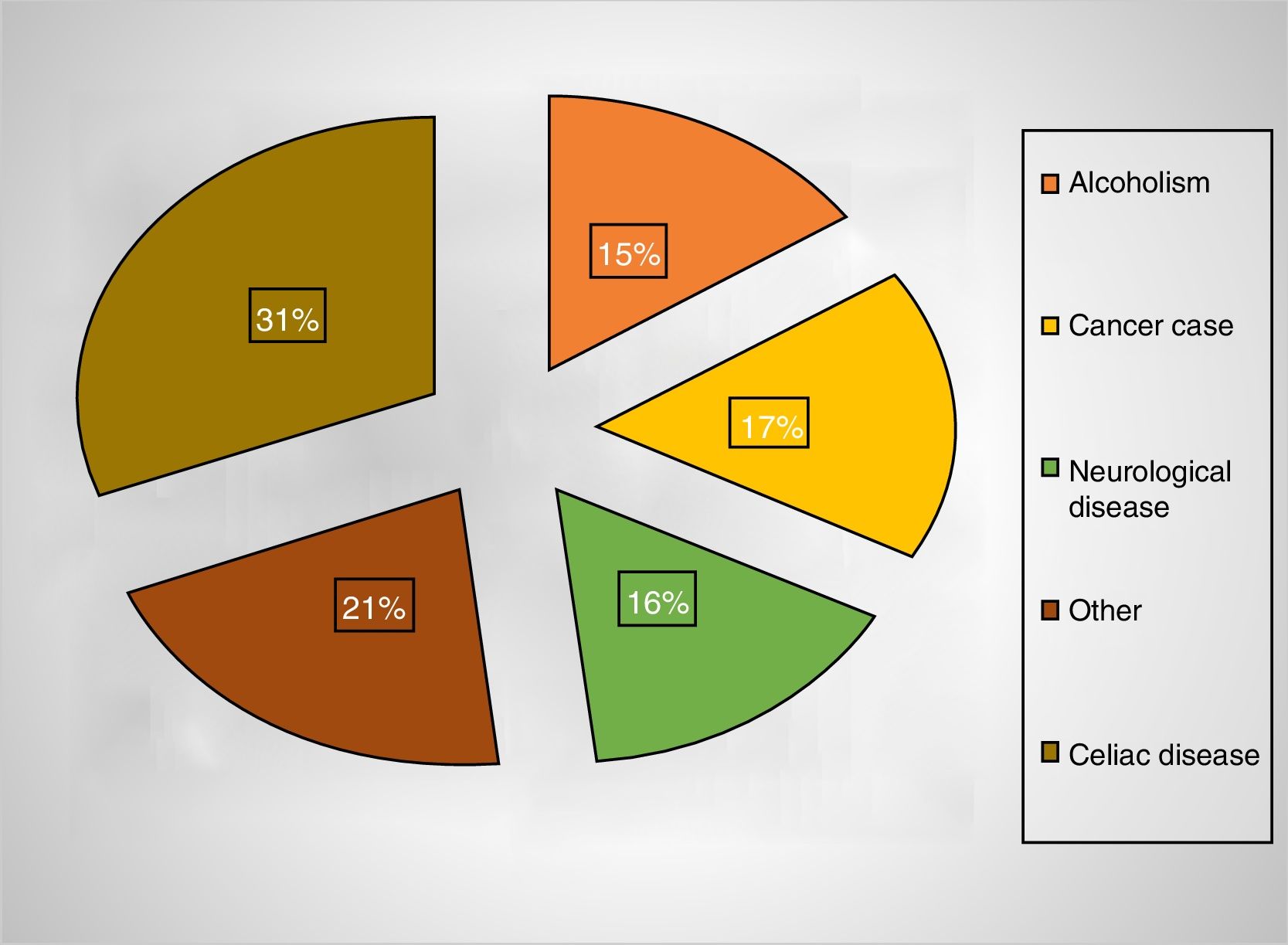
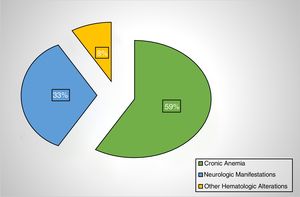
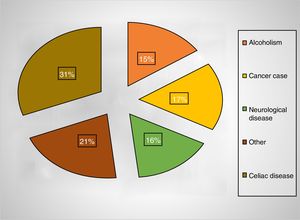
Of the patients with folate deficiency, the measurements were requested for chronic anemia in 59%, neurological manifestations in 33%, and other hematological alterations in 9% (Figure 2). Of the patients with chronic anemia, 23% had high MCVs and 50% had celiac disease. A high MCV was observed in 17% of the cases with folic acid deficiency vs. 5% in the group without deficiency (p-value<0.05). The medical conditions most commonly associated with folate deficiency were celiac disease (31%) and alcoholism (15%) (Figure 3). Interestingly, in only 55% of the cases, the attending physicians provided folate supplements to their patients with deficiency.
DiscussionIn the study population, the prevalence of folic acid deficiency after the supplementation of wheat flour was 1.7%. There was a significant decrease in folate deficiency and risk of a deficiency following supplementation. Our findings coincide with other studies,11 in which a marked reduction was observed in pregnant women and women of childbearing age. A decrease in neural tube defects associated with this supplementation was also described; for example, neural tube defects were reduced by 56% in Argentina and 43% in Chile. In addition, the study by Brown et al.17 in an Australian population showed a reduction in folate deficiency of 77% after flour supplementation.
The prevalence of folate deficiency in the present study was markedly reduced after supplementation, but slightly higher than in other recent publications, in which it ranged from 0.06 to 0.1%.10,18 This difference could be due to the different populations studied. In addition, it could be because the individuals in Anglo-Saxon studies might have received larger amounts of dietary folic acid or they could have been exposed to flour fortification for longer periods.
Although possible, it seems unlikely that a change in the criteria for requesting serum folate levels might explain the striking differences in the folate levels after wheat flour supplementation. Staff members from the Internal Medicine, Hematology, and Neurology Departments, the professionals who most requested tests in the study hospital, did not change substantially during the study period. The fact that folate levels remained stable after 2007 with minor deviations suggests that the requesting pattern would not potentially explain the findings of the present study.
In addition, the change in the system for determining the serum folate levels after 2010 would not explain the notable reduction in folate deficiency after supplementation. The reference levels for the serum folate were identical for the Cobas® and Architect Integrated System® chemiluminescence assays, and a recent publication found that differences in the methods were seen exceeding acceptable bias for thyroid-stimulating hormone, free thyroxine, cobalamin (vitamin B12), and ferritin, but not for folate mesurements.19
According to these results and those of other studies, the supplementation of wheat flour with folic acid significantly reduced serum folate deficiency, and thus, supplementation is an example of the very short-term success of a nutritional intervention measure in public health focused on the population. Patients at greater susceptibility to deficiency are children, over 50-year-old patients, celiac disease patients, alcoholics, and patients with cancer, in whom both the disease itself and the treatment can decrease serum folate levels.20,21
In 59% of the cases, the measurement was requested to evaluate chronic anemia; in this group, only 17% had high MCVs. Despite the deficiency, health professionals opted for supplementation in only half the cases, without having a cohesive criterion for such supplementation.
Folate measurement is routinely recommended in the evaluation of macrocytic anemia.8 Macrocytosis, with or without anemia, is a frequent finding in the daily clinical practice; however, a serum folate measurement is considered to be an indicator of recent folate intake. However, it is not possible to differentiate between a transient reduction in the dietary intake and a chronic deficiency with a single measurement.
The folate levels in erythrocytes are considered better indicators of true deposits; however, these may be normal in the early stages of deficiency.22 The erythrocyte folate concentrations respond slowly to variations in the intake, because the erythrocytes only accumulate folate during erythropoiesis. Consequently, the erythrocyte concentrations are useful as indicators of the long-term folate nutritional status.23
Some studies in developed countries have found low incidences of abnormal folate results, and they concluded that screening for folate deficiency is not cost-effective.8,17 Although we did not perform a formal cost-effective analysis, our data suggest the same conclusion.
This study had some limitations. First, the retrospective observational design based on the analysis of electronic medical records did not allow the collection of many clinical variables. Moreover, we did not assess the causes for requesting the measurement of folate in the pre-supplementation group. In the study institution, electronic medical records were not available before 2008; therefore, evaluating the reasons to request a test of folate levels in the pre-supplementation group would be very difficult, if not impossible. Second, the population assisted at the Hospital Privado de Córdoba may not represent the entire Argentinian population, with a bias toward the social strata with greater economic resources and a lower risk of malnutrition. Third, there are no systematic studies on the direct measurement of folic acid content in bread, although the National Administration of Medicines, Food, and Medical Technology (ANMAT) in Argentina routinely carries out controls at flour mills. Furthermore, the bread and wheat flour consumption per patient during the study period is not known.
Given the low prevalence of folic acid deficiency observed in this and other similar studies, and the lack of changes in the clinical management of many of these patients, we conclude that the routine measurement of serum folic acid is of limited clinical use.
Conflicts of interestThe authors declare no conflicts of interest.