Recently, a small peptide called Hepcidin, was found to have an important role in regulating the iron metabolism in anemia of chronic disease (ACD) patients. Hepcidin is regulated by a variety of conditions at the transcriptional level. Therefore, our study aims to predict the level of hepcidin serum using inflammation markers and iron indicators in patients afflicted with ACD and observe how this severity of inflammation separated the level of interleukin-6 (IL-6), as well the as hepcidin level.
MethodsA cross-sectional data analysis was conducted on 80 ACD adult patients treated at the Sanglah Teaching Hospital in Bali, Indonesia. We used hepcidin serum and several markers, such as the hemoglobin level, inflammation markers, renal function tests, IL-6, and iron indicators, to predict the hepcidin level.
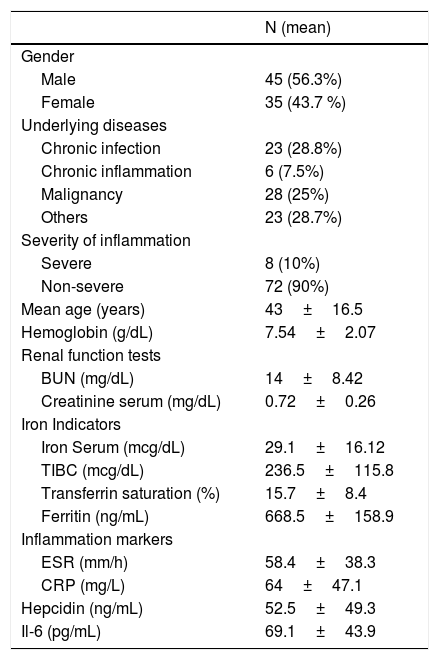
ResultsThis study recruited 80 ACD patients, comprising 45 men (56.3%) and 35 women (43.7%). The mean age of the participants was 43±16.5 years. Only IL-6, ferritin and serum creatinine correlate significantly with serum hepcidin from seven variables that were previously eligible to enter the analysis. This study found the model to predict the hepcidin level using IL-6 ferritin and the creatinine level as the hepcidin level (predicted)=−23.76+0.396 (IL 6)+0.448 (ferritin)+0.310 (creatinine).
ConclusionThis study has revealed that the creatinine level, ferritin and IL-6 can be used to predict the hepcidin level in patients with anemia of chronic disease. It is to be hoped that further cohort studies can validate our formula to predict the hepcidin level.
Anemia of chronic disease (ACD) is classically associated with a triad of inflammation, infection, and neoplasm. Other diseases such as anemia of older adults, diabetes mellitus, severe trauma, congestive heart failure and anemia with chronic kidney disease also shared characteristics similar to those of ACD, for example, normochromic normocytic anemia, hyporegenerative anemia, hypoferremia, and mild and moderate degrees of anemia. In certain conditions such as in severe inflammation, less than 25% of ACD cases also present with hypochromic microcytic anemia. Although the slight red blood cell (RBC) lifespan reduction due to increased activities of activated macrophages also contributes to the development of anemia in ACD, actual ACD should be seen as a spectrum of acute to chronic immune activation in response to injury or inflammation because all of these have a similar and common underlying mechanism in the end.1
Despite an increased or normal level of ferritin, there are significant reductions in serum iron and the iron binding capacity of transferrin during infection or inflammation. These reductions are caused either by the inflammatory response of ferritin as one of the positive acute phase reactants or by iron loading in the monocyte-phagocytic system (MPS).2
Recently, hepcidin, a small peptide produced by liver cells and other immune active cells, was found to have an important role in regulating the iron metabolism in ACD patient.3 This small peptide is essential for iron hemostasis, as its absence or overproduction can cause devastating health consequences, including iron siderosis in vital organs (heart, liver, and pancreas). In normal conditions, hepcidin binds to ferroportin, its only receptor, and then the binding will be degraded to prevent iron export from duodenal enterocytes, macrophages and liver cells to reduce the availability of adequate serum iron for erythropoiesis.3 In addition, hepcidin also reduces apical uptake of iron in the duodenum. The exact mechanism of how hepcidin reduces the apical uptake of iron is not fully understood. It was hypothesized that by degrading the binding between hepcidin and ferroportin, hepcidin will decrease the synthesis of apical divalent metal transporters-1 (DMT1) and other apical iron transporters, including the heme transporter. As a result, the apical uptake of iron in the duodenum will decrease.3 In other words, the low level of hepcidin will increase iron absorption in the duodenum and iron export into circulation for erythropoiesis. Furthermore, a high level of hepcidin in the urine is also seen in patients with sepsis.4
Hepcidin is regulated by a variety of conditions at the transcriptional level, not only by the inflammation process by its major mediator, interleukin 6 (IL-6), but also by the iron level and erythropoietic response. However, recent studies showed that the non-IL-6 dependent mechanism also modulated hepcidin expression. In ACD patients, the hepcidin level has been reported to cause hypoferremia and iron loading in macrophages, due to the inflammation-induced pro-inflammatory cytokine cascade, such as IL-6, IL-1 and tumor necerosis factor alpha (TNFα).5 Furthermore, studies in animals and humans have suggested that hepcidin is the major significant active player in iron disorders seen in ACD patients. This fact is supported by the study of Papadaline et al., that reported that TNFα antibody therapy in patients with rheumatoid arthritis had alleviated anemia and reduced the IL-6 level. An injection of anti-IL-6 receptor antibody tocilizumab in Still’s disease resulted in an improvement in the clinical condition, followed by the reduction of the C-reactive protein (CRP) level and ferritin. In ACD patients, the IL-6 level significantly correlated with hepcidin detected in circulating macrophages and with decreased ferroportin expression.6 In 65 lymphoma patients, the hepcidin level was significantly higher than in the controls and was associated with IL-6 level. Hepcidin and IL-6 were significantly higher in the aggressive type of lymphoma. In an animal study in which knockout mice had a complete lack of hepcidin, the authors stated that hepcidin inhibited iron uptake from the small intestine and blocked iron export from macrophages.
Therefore, we aim to predict the level of serum hepcidin using inflammation markers and iron indicators in patients with ACD. We also aim to examine the mechanism of how this severity of inflammation separated the level of IL-6, as well as the hepcidin level.
MethodsThis was a cross-sectional data analysis on 80 adult ACD patients treated at the Sanglah Teaching Hospital in Bali, Indonesia. Basic demographic data along with underlying diseases and severity of inflammation were collected by means of a questionnaire.
The eligibility criteria were as follows: (1) Men aged 18 years or older, (2) afflicted with ACD, (3) willing to provide blood samples for serum hepcidin, hemoglobin level, inflammation markers (e.g., the erythrocyte sedimentation rate (ESR) and CRP), renal function tests (such as blood urea nitrogen and serum creatinine), interleukin-6 (IL-6), and iron indicators (such as the serum iron and total iron binding capacity (TIBC)), transferrin saturation, and ferritin) and (4) able and willing to provide written informed consent. ACD was defined based on the hemoglobin level (HB<13g/dL in men and HB<12g/dL in women), morphologically normochromic or hypochromic, transferrin saturation less than 20%, and ferritin >100mcg/L, the presence of infection, inflammation, and malignancy, such as the HIV/AIDS infection, tuberculosis, rheumatoid arthritis, systemic lupus erythematosus, malignant lymphoma, urogenital malignancy and gastrointestinal cancer. The study was approved by the institutional review board of the Sanglah Teaching Hospital. The exclusion criteria were: iron deficiency anemia, patient on iron therapy and acute bleeding episode within 2 months before study began. The severity of the inflammatory status was considered when the CRP level ≥60mg/L and the ESR≥100mm/h.7,8
Hematologic parameters were measured using a flow cytometry semiconductor laser ray with the length of 663nm, in which cells were analyzed based on Forward Scattered Light (FSC), Side Scattered Light (SSC) and Side Fluorescent Light (SFL) on the Sysmax XN. The serum iron and TIBC were measured with the Roche/Hitachi Cobas c systems, an analysis based on the colorimetric assay. The CRP was also determined using the Roche/Hitachi Cobas c systems, this assay being based on the principle of particle enhanced immunological agglutination. The serum ferritin was determined by the IMMULITE 2000, which is a solid phase two-site chemiluminescent immunometric assay. Lipemic samples were avoided using an ultracentrifuge. The DRG hepcidin 25 (bioactive) HS ELISA kit was used to measure the serum hepcidin, based on competitive binding principles in which the enzyme conjugate (hepcidin 25 biotin) competes with hepcidin 25. The serum IL-6 was measured using the 4.0h solid-phase ELISA, the Quantikine HS Human IL-6 immunoassay. This assay employs the quantitative sandwich enzyme immunoassay technique.
Data were analyzed by the SPSS 21.0 for windows, in which the correlation of the hepcidin level with the iron indicator, IL-6 and CRP, using either the Pearson product of moment or the Spearman, based on the normality of data distribution. The relation of hepcidin with the severity of the inflammation status, using the independent t-test, as well as the predictive of the hepcidin level through stepwise multiple regression analysis, were also analyzed. The significance level of less than 0.05 was accepted.
ResultsThis study recruited 80 ACD patients, comprising 45 men (56.3%) and 35 women (43.7%). The mean age of the participants was 43±16.5 years. The remained study characteristic can be seen in Table 1 (Table 2).
Characteristics of respondents.
| N (mean) | |
|---|---|
| Gender | |
| Male | 45 (56.3%) |
| Female | 35 (43.7 %) |
| Underlying diseases | |
| Chronic infection | 23 (28.8%) |
| Chronic inflammation | 6 (7.5%) |
| Malignancy | 28 (25%) |
| Others | 23 (28.7%) |
| Severity of inflammation | |
| Severe | 8 (10%) |
| Non-severe | 72 (90%) |
| Mean age (years) | 43±16.5 |
| Hemoglobin (g/dL) | 7.54±2.07 |
| Renal function tests | |
| BUN (mg/dL) | 14±8.42 |
| Creatinine serum (mg/dL) | 0.72±0.26 |
| Iron Indicators | |
| Iron Serum (mcg/dL) | 29.1±16.12 |
| TIBC (mcg/dL) | 236.5±115.8 |
| Transferrin saturation (%) | 15.7±8.4 |
| Ferritin (ng/mL) | 668.5±158.9 |
| Inflammation markers | |
| ESR (mm/h) | 58.4±38.3 |
| CRP (mg/L) | 64±47.1 |
| Hepcidin (ng/mL) | 52.5±49.3 |
| Il-6 (pg/mL) | 69.1±43.9 |
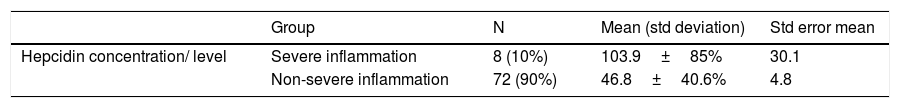
Relation of the hepcidin level with the severity of inflammation.
| Group | N | Mean (std deviation) | Std error mean | |
|---|---|---|---|---|
| Hepcidin concentration/ level | Severe inflammation | 8 (10%) | 103.9±85% | 30.1 |
| Non-severe inflammation | 72 (90%) | 46.8±40.6% | 4.8 |
Sig. of level test for equality of variances equal variances assumed; p=0.008.
Sig. of the t-test for equality of variances equal variances assumed; p=0.001.
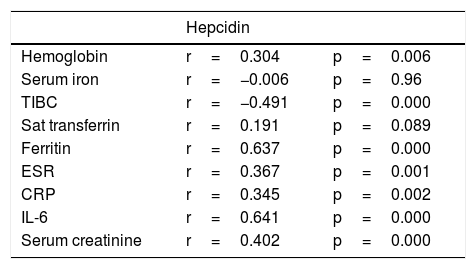
This study found that the hepcidin level becomes significantly higher when the inflammation becomes more severe (103.9±85.2), compared to the non-severe inflammation condition (46.8±40.6) with p=0.001. On the correlation matrix, almost all inflammation markers and iron indicators correlate significantly with hepcidin, as shown in Table 3, except for the iron serum and transferrin saturation (Table 4).
Correlation of hepcidin with iron indicators and inflammation markers.
| Hepcidin | ||
|---|---|---|
| Hemoglobin | r=0.304 | p=0.006 |
| Serum iron | r=−0.006 | p=0.96 |
| TIBC | r=−0.491 | p=0.000 |
| Sat transferrin | r=0.191 | p=0.089 |
| Ferritin | r=0.637 | p=0.000 |
| ESR | r=0.367 | p=0.001 |
| CRP | r=0.345 | p=0.002 |
| IL-6 | r=0.641 | p=0.000 |
| Serum creatinine | r=0.402 | p=0.000 |
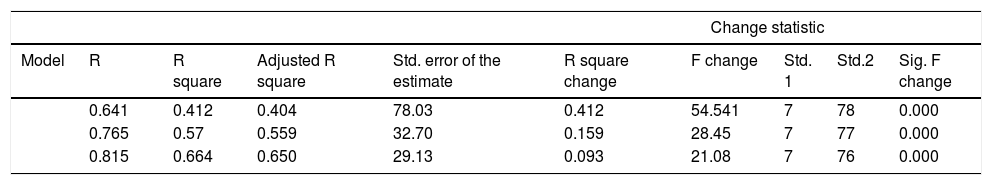
Stepwise multiple regression analysis for the hepcidin level.
| Change statistic | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | R | R square | Adjusted R square | Std. error of the estimate | R square change | F change | Std. 1 | Std.2 | Sig. F change |
| 0.641 | 0.412 | 0.404 | 78.03 | 0.412 | 54.541 | 7 | 78 | 0.000 | |
| 0.765 | 0.57 | 0.559 | 32.70 | 0.159 | 28.45 | 7 | 77 | 0.000 | |
| 0.815 | 0.664 | 0.650 | 29.13 | 0.093 | 21.08 | 7 | 76 | 0.000 | |
| Model | Unstandardized coefficients | Standardized coefficients | T | Sig | |
|---|---|---|---|---|---|
| B. | Std. error | Beta | |||
| 1. Constant | 29.28 | 5.29 | 5.54 | <0.001 | |
| IL 6 | 0.34 | 0.05 | 0.641 | 7.39 | <0.001 |
| 2. Constant | 17.04 | 5.10 | 3.34 | 0.001 | |
| IL 6 | 0.24 | 0.04 | 0.451 | 5.44 | <0.001 |
| Ferritin | 0.003 | 0.01 | 0.442 | 5.33 | <0.001 |
| 3. Constant | −23.76 | 9.98 | −2.38 | 0.020 | |
| IL 6 | 0.21 | 0.04 | 0.346 | 5.30 | <0.001 |
| Ferritin | 0.03 | 0.01 | 0.448 | 6.07 | <0.001 |
| Creatinine | 58.78 | 12.80 | 0.310 | 460. | <0.001 |
After stepwise regression analysis had been done, only the IL-6, ferritin and serum creatinine correlate significantly with the serum hepcidin from 7 variables that were previously eligible for the analysis. This study found the model to predict the hepcidin level, using IL-6 ferritin and creatinine levels, for example:
Hepcidin level (predicted)=−23.76+0.346 (IL 6)+0.448 (ferritin)+0.310 (creatinine).
Additionally, this model was able to predict approximately 65% of the hepcidin level when IL 6, ferritin and creatinine were used, while the remaining 35% of the hepcidin level was influenced by other factors. Serum creatinine had the least impact on the hepcidin level, compared to ferritin and IL 6, whereas IL 6 had the strongest impact on the predicting of the hepcidin level, as can be observed in the R square change statistics.
DiscussionAnemia of chronic disease is commonly associated with infection, inflammation, and malignancy. However, recent literature has noted that other conditions, such as severe acute illness, massive trauma, diabetes mellitus, kidney disease, congestive heart failure and anemia in older adults, can resemble pathogenesis and laboratory results of classic ACD. Therefore, it is important to recognize the hallmark of ACD, the hypoproliferative state. In the hypoploriferative state, the red blood cell (RBC) production will fall due to the inadequate supply of iron to the bone marrow. In turn, the combination of this inadequacy and the increased level of the iron store (ferritin) stimulates the production of hepcidin by hepatocytes. Furthermore, the underlying infection and inflammation stimulate cytokine release, such as IL-6, TNFα, IL-1, by activated macrophages. The cytokine release will then trigger a cascade reaction and stimulate the lymphocytes to produce interferon (IFN).9 Moreover, hepcidin production induced by IL-6 will inhibit iron absorption by duodenal cells and block iron export into the blood circulation. The inhibition and blockage of iron will reduce plasma iron (hypoferremia).6 Hepcidin, or the hepatic antimicrobial peptide (HAMP), is also produced by hepatocytes in response to injury or infection. This protein, originally described by Ganz et al.3, has antimicrobial activities. Nonetheless, recent studies have confirmed that hepcidin will limit iron availability, which is important for pathogens to grow. This restriction occurs through the inhibition of iron absorption by duodenal enterocytes and iron recycling by the monocyte-phagocytic system (MPS). Furthermore, the internalization and degradation of ferroportin, a known iron exporter in the human body, will cause hypoferremia.
Chronic inflammation will occur when the immune response cannot completely eliminate the offending pathogens in this condition in which there are the sustained stimulation of cytokine release and hepcidin up-regulation. The increase in the hepcidin level in response to pathogen infection is not equal across every infection, for example, in patients with AIDS/HIV infection, hepcidin is increased, but not in hepatitis B and C.10 It is thought that hepcidin-induced hypoferremia is determined by the type of pathogen, site of infection and typical immune response.11 During inflammation and infection, the activated macrophages will unleash cytokine networks, which contain IL-6. The quantity of IL-6 induced by hepcidin secretion depends on the severity of the inflammation.12 In contrast, when the inflammation is resolved, the erythroferrone precursor (ERFE) will facilitate hepcidin down-regulation and will accelerate the recovery process of the organism from iron disorders during the inflammatory process.11
In our study, we found that the hepcidin level was significantly associated with the severity of the inflammation (p=0.001). Due to the variability of underlying diseases, we use the ESR >100mm/h and the CRP level ≥60mg/L as markers for severe inflammation. The ESR >100mm/h was also found by Fincher and Page as the most common marker in infection.8 Ter Berg et al. performed a study to determine the CRP level during disease exacerbation and infection in systemic lupus erythematosus (SLE) patients.7 They found that a CRP >60mg/L (6mg/dL) was found in all patients with infection and exacerbation.8 The CRP is a positive acute phase reactant associated with acute and chronic inflammations that are caused by various different diseases, for example, infection, non-infection inflammatory disorders and neoplasm. Normal references, in order to be unequivocally accepted, depends on the laboratory, ages, gender, and race. It is best to consider significant clinical inflammation when the level of CRP is greater than 10mg/L (1mg/dL). A CRP of 3–10mg/L (0.3–1mg/dL) is suggestive of what is known as low-grade inflammation.13
The association between hepcidin and the disease severity was also reported by several other studies. Oustamanolakis et al. have examined 160 inflammatory bowel disease (IBD) patients and 102 healthy controls.14 A positive correlation was found between hepcidin and the disease activity of ulcerative colitis (r=0.36; p<0.05).14 This positive association remained significant after multivariate analysis was performed.14 The disease activity of ulcerative colitis was determined using the simple clinical colitis activity index (SCCAI) greater than 3.14 Although no strong explanation was provided, it was hypothesized that it may be attributed to the sustained stimulation of cytokine release, especially IL-6 families during active inflammation that trigger hepcidin production. Hepcidin was also found to be positively correlated with the injury severity score (ISS) in the study of 150 patients with severe trauma, as well as in 92 consecutive patients admitted because of sepsis.15,16 In the severe trauma cases, the hepcidin level was found high on admission. The hepcidin level became even higher, as the systemic inflammation response syndrome became more severe.15,16 A study conducted on 185 patients with rheumatoid arthritis reported that the hepcidin level significantly correlated with the disease activity score (DAS 28) >5.1. In this study, the DAS 28 was calculated using clinical and laboratory data, the ESR and CRP rates, the IgM Rheumatoid Factor (RF) titer and the anti-cyclic citrullinated peptide (anti-CCP) antibody rate. Patients with a DAS 28 score >5.1 were considered to have active disease. Another study by Darton et al. performed experimental human typhoid infection with 50 study participants.17 The serum hepcidin, along with other iron indicators and inflammatory markers, was evaluated during the 40 days following the typhoid challenge. They reported that the hepcidin level was found to be significantly higher during the acute typhoid infection than at the baseline and that it was higher when the fever increased and during inflammation, p<0.0001.
In all these studies, it was assumed that the sustained cytokine release from activated macrophages especially IL-6, would lead to an upregulated hepcidin production during the progression of the inflammation. Therefore, it is to be hoped that hepcidin will show a significant correlation with disease activities, except in the situation where the impending pathogen or injury can be eliminated following the release of the ERFE protein. This protein can inhibit the signaling pathway that stimulates the hepcidin production.11
There are 3 major hepcidin regulators at the transcriptional level, namely iron, inflammation, and erythropoietic activities. For the inflammation-induced hepcidin production, the best described and probable primary modulator is IL-6, among other non-IL6-dependent pathways.4,18 Patients with severe infection, sepsis, IBD, rheumatoid arthritis, SLE, multiple myeloma, non-Hodgkin lymphoma and breast cancer, will present increased hepcidin expression.
Our study also found that the hepcidin level was significantly correlated with the serum iron, transferrin saturation, ferritin, ESR, CRP and IL-6 (p<0.05). Interestingly, our stepwise regression analysis found that only IL-6, ferritin and serum creatinine remained significantly correlated with adjusted R square 0.65 and p<0.01. This study found the model to predict the hepcidin level using the IL-6 ferritin and creatinine levels, for example:
This model can predict approximately 65% of the hepcidin level when IL-6, ferritin and creatinine are used, while the remaining 35 % of hepcidin level was influenced by other factors. The serum creatinine has the weakest impact on the hepcidin level, compared to ferritin and IL-6, whereas IL-6 has the strongest impact on the prediction of the hepcidin level, as can be observed in the R square change statistics. This finding is supported by the study by Ashby et al., demonstrating that serum hepcidin in the multivariate analysis is inversely correlated with the glomerular filtration rate (GFR) in adults with stage II-IV IIIV chronic kidney disease (CKD).19 Furthermore, the hemodialysis group had the highest level of hepcidin.19 The study also noted that the hepcidin level increased 2–4 fold in pre-dialysis patients and 6–9 fold in hemodialysis patients.19 This data implies that patients with decreased kidney function and high creatinine levels will have a high hepcidin level. This high level may have occurred through diminished pro-inflammatory cytokine clearance12,20, such as IL-6. These cytokines were found to be inversely correlated with the creatinine clearance.21 A study on rats reported that the half-life of pro-inflammatory cytokines, including the TNFα and IL-1, was longer in those with decreased kidney function than in those with normal kidney function.22 In patients with CKD, conditions such as volume overload with endotoxemia, oxidative stress, low level of antioxidants and presence of comorbidities, also play their role in stimulating the inflammation process. In end stage renal disease (ESRD) patients, the inflammation process has proved to be more prominent due to frequent exposure to bio-incompatible membranes, back diffusion of contaminants and chronic access devices. Furthermore, these factors may provoke latent infection by developing a biofilm that finally induces hepcidin secretion.23
We found a strong positive correlation between ferritin and hepcidin levels. Our result is in line with the study on Crohn’s disease conducted by Basseri et al.24 They showed a strong positive correlation between ferritin and hepcidin levels (r=0.723, p=0.0015).24 Ashby et al. also found a strong correlation between ferritin and hepcidin levels in all study groups of CKD patients (r=0.673, p<0.0001). A study on 247 IBD patients at a Swiss IBD study center reported that regardless of the inflammatory status, patients with ferritin less than 30mcg/L had a significantly lower hepcidin level than those with ferritin >30mcg/L, which significantly correlated, with r=0.491 and p<0.001. However, in the multivariate analysis, only ferritin correlated significantly (p=0.015). The study suggested that the iron store was the prominent trigger to stimulate hepcidin production.25 Another study on IBD patients showed that the serum hepcidin and the pro-hepcidin were significantly correlated with the ferritin level (r=0.34, p=0.007 and r=−0.21, p=0.04, respectively). This association remained after adjusting with other variables (p=0.0008).14 At present, it is believed that during inflammation, most of the ferritin that circulates in the bloodstream is released by activated macrophages.26 Hepcidin-induced ferroportin degradation will lead to intracellular iron sequestration. As a result, more ferritin will be released to the circulation, along with inflammation-induced hepcidin secretion. This condition explains why the serum ferritin level has a strong correlation with hepcidin in patients with inflammation. One of the possible reasons is that hepcidin or ferritin is considered a positive acute phase reactant, a glycosylated protein produced almost exclusively by hepatocytes. These proteins will be increased by at least 25% during inflammation, as they are essential in the metabolic disorder restoration process.27
During the inflammation process, hepcidin secretion seems to be less sensitive to the iron state, for example in anemia or inflammation. If the iron level is low, hepcidin should be suppressed. In inflammation, the hepcidin level is still high because of the high level of inflammatory cytokines such as IL-6. This corroborated our study, in which IL-6 was significantly correlated with hepcidin even after multivariate regression analysis was performed (p<0.000). The importance of IL-6 as a primary mediator is also being suggested in numerous studies, both with animal and human subjects. In animal studies, the IL-6 hepcidin pathway was shown when turpentine was injected into the cells of IL-6 knockout mice. The hepcidin response was also lost when lipopolysaccharides (LPS), combined with the IL-6 antibody, were injected into cultured hepatocytes. As a result, the expression of hepcidin mRNA was blunted.2 A study on Castleman’s disease, a well-known inflammatory disease, treated with tocilizumab (antibody to the IL-6 receptor) has shown that a low level of hepcidin was followed by the normalization of iron indicators and an improving clinical condition. It links IL-6 to inflammatory-induced hepcidin production.28 A case-control study on 65 Hodgkin Lymphoma patients reported that IL-6 was significantly higher than in the controls (p<0.05). Furthermore, IL-6 was found to have a significant positive correlation with the hepcidin level. These human and animal studies strongly suggest that inflammatory induced IL-6 is necessary to produce more hepcidin. The extra production of hepcidin is necessary to assist the body in retaining iron. This detaining process can occur by sequestrating iron intracellularly, depriving the iron-dependent pathogen of iron and eliminating inflammation stimulation. Although the mechanism by which IL-6 molecularly signals the production of hepcidin is not fully understood, IL-6 appears to play its role by binding to its surface the IL-6 receptor which stimulates the downstream cascade leading to JAK-2 activation. Furthermore, the binding of phosphorylatedSTAT3 with the STAT3 response element activates the hepcidin transcription.29 In addition to inflammation, iron and erythropoietic activities also regulate the hepcidin expression. Serum iron, with its transporter transferrin after binding to the transferrin receptor, will stimulate downstream signaling of BMP SMAD4W, activate the upstream response element of the hepcidin gene and, finally, stimulate hepcidin secretion. Hepcidin secretion inhibits duodenal iron absorption and degrades the only iron exporter, ferroportin, leading to hypoferremia.3
The limitation of our study is that we used Hepcidin 25 ELIZA for hepcidin measurement. Hepcidin 25 ELIZA depends on a very specific hepcidin antibody that is used as a reference standard for synthetic hepcidin. In order to avoid this limitation, mass spectrometry can be used to measure hepcidin. By using mass spectrometry, the mass of hepcidin active 25 amino acid can be examined and compared to the initial standard. However, the spectrometry requires an internal isotope hepcidin standard that is not widely available. In addition, it is affected by the manifold lower level diurnal variation in the morning, increasing in the afternoon.30 Furthermore, there was no international standardized value. Other than mass spectrometry, a more sensitive assay is underway and while awaiting the assay validation, the present tools nonetheless remain essentially experimental.18
ConclusionThis study found that hepcidin seems to play its prominent role in iron metabolism, as hepcidin significantly correlates with almost all inflammation markers and iron indicators, such as serum iron, transferrin (TIBC), ferritin, ESR, CRP, IL-6 and creatinine. Our study has revealed that only the creatinine, ferritin, and IL-6 levels can significantly be used to predict the level of hepcidin in patients with the anemia of chronic disease.
Conflicts of interestThe authors declare no conflicts of interest.