The allogeneic hematopoietic stem cell transplantation (HSCT) conditioning protocol and the use of immunosuppressant drugs make patients more susceptible to bacterial, fungal, and viral infections.1,2 Infections occurring in the oral cavity may be single or multiple and often acquire characteristics different from those commonly found in immunocompetent patients, making the differential clinical diagnosis hard to establish.1,2
After HSCT, the hemolymphopoietic system of the recipient undergoes a period of secondary toxicity that manifests as aplasia and may last several weeks, with medullary and immunological recovery depending on the type of graft source.3 Approximately, neutrophil recovery occurs within 2 weeks in individuals who received peripheral blood as a graft source, within 3 weeks in those who received bone marrow as a graft source, and within 4 weeks in patients who received umbilical cord blood as a graft source.3 Moreover, recovery of neutrophils, monocytes, and natural killer (NK) cells (whose recovery occurs within 1–2 months) is commonly followed by the recovery of platelets and erythrocytes and then by the recovery of B lymphocytes (B cells) and T lymphocytes (T cells). More specifically, CD8+T cells take 2–8 months to reach normal rates, whereas B cells require 6 months, and CD4+T cells need 12 months.3
In allogeneic HSCT, immunosuppressive drugs are administered to prevent or treat graft versus host disease (GVHD). These drugs increase the chances of individuals having some infections because of low cellular immunity. In order to avoid infections in the oral cavity, it is extremely important that patients be advised by their dentists about the importance of good oral hygiene to avoid short- and long-term infections, especially in the first year after HSCT.3
Considering general infections affecting patients after HSCT, candidiasis is one of the most frequent fungal infection, while Herpes simplex (HSV) and Varicella-zoster (VZV) appear as the most frequent viral infections, affecting 70% and 10–30% of them, respectively.1 Gram-positive bacteria is the most frequent bacterial infection.1,4,5
The occurrence of acute graft-versus-host disease (aGVHD) or chronic graft-versus-host disease (cGVHD) after HSCT is influenced by several factors, including the patient's age, whether the patient and the donor are positive or negative for the Cytomegalovirus (CMV) serology test, the type of graft, the conditioning chemotherapy and the number of stem cells (CD34+) and T lymphocytes contained in the inoculum.1,4 The main causes of morbidity and mortality in patients who undergo allogeneic HSCT are relapse of the underlying disease, graft failure, infections and acute or chronic GVHD.1,2 When associated with slow medullary recovery, acute or chronic graft-versus-acute host disease and systemic immunosuppression make the patient more susceptible to opportunistic infections.1,2,4
In view of this situation, it is extremely important that the patients be regularly submitted to oral exams and dentists be aware of the possible oral complications after HSCT. Therefore, adequate monitoring is possible, with early diagnosis and intervention, when necessary.
Case reportA 28-year-old male patient with the diagnosis of chronic myeloid leukemia (CML) underwent an unrelated donor allogeneic hematopoietic stem cell (bone marrow) transplantation on March 2, 2016. In the dental assessment immediately before the HSCT, the patient had no infection in the oral cavity and had a positive CMV immunoglobulin G (IgG) result. Conditioning was performed with cyclophosphamide+busulfan+antithymocyte globulin (ATG), and prophylaxis for GVHD was performed with cyclosporine. The patient presented grade III acute GVHD on D+44, acute ocular GVHD on D+47, and acute GVHD of the gastrointestinal tract on D+71. After bone marrow acceptance and clinical stability, the patient was discharged from the hospital, remaining under outpatient follow-up. The patient received cyclosporine 300mg/day from D+36 to D+52. The patient has started tacrolimus and methylprednisone intake on D+67 and continued with this regimen until D+108. The aGVHD showed resolution on D+112.
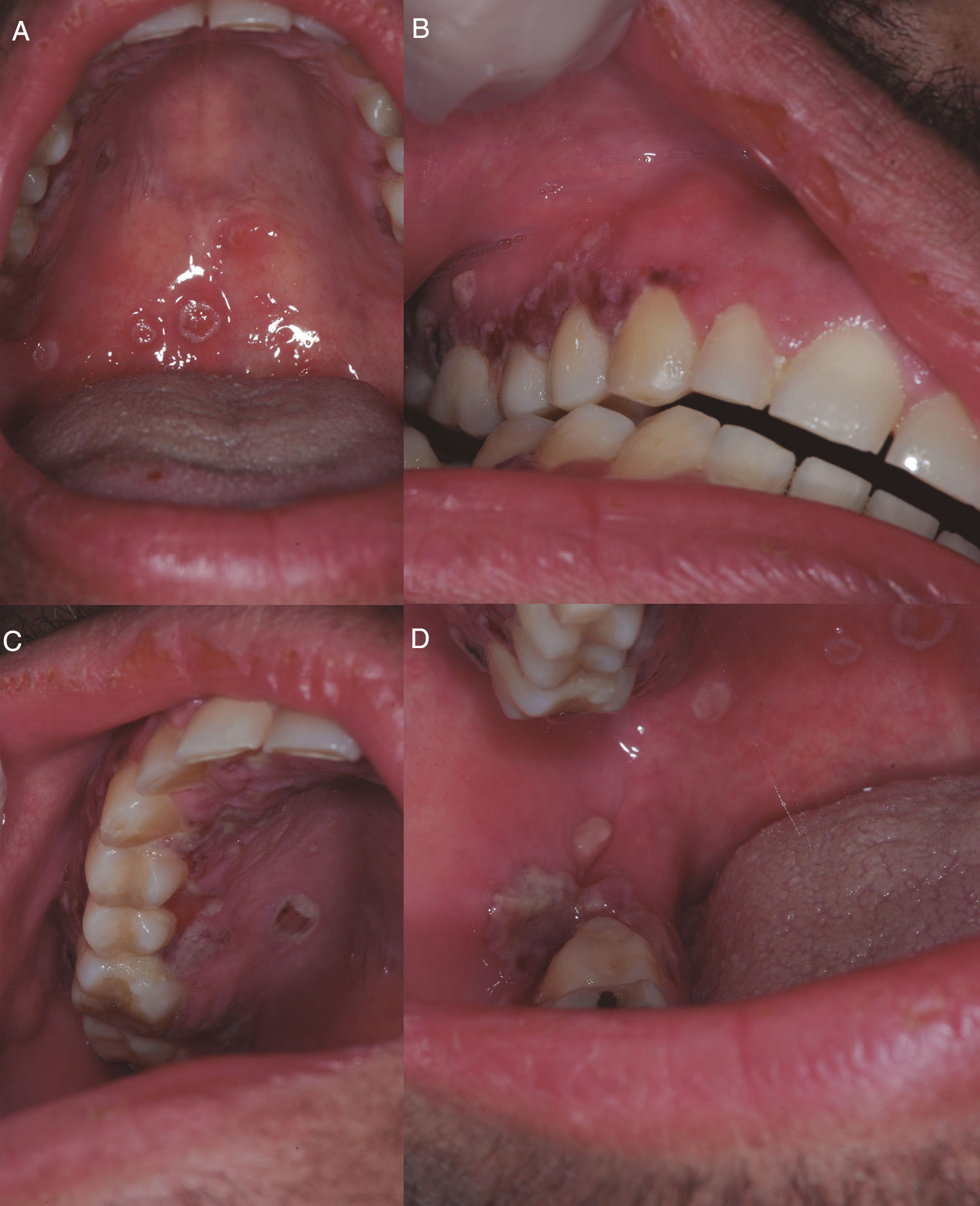
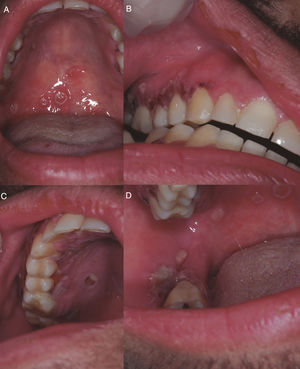
On D+218 (10/6/2016), the patient attended the dental outpatient department complaining of odynophagia and the physical examination revealed multiple clustered and isolated ulcers with irregular and raised borders on the soft and hard palate on the right side, on the right buccal mucosa and on the gingival margin of the right retromolar trigone area. In addition, there were areas of erythema and gingival overgrowth of fibrous consistency, with areas of necrosis on the upper right gingival margin on the palatine surface. The upper gingival margin on the vestibular surface was dark red in color, extending from the canine to the second molar area (Figure 1). The lesions on the free gingival margins exhibited a punched-out aspect (Figure 1B). The other areas of the oral cavity were intact; the patient presented good oral hygiene and good salivary flow (sialometry=0.96mL/min). The patient did not present skin lesions on the face.
(A) Ulcers with irregular and raised borders on the soft palate. (B) Ulcers with irregular and raised borders on gingival margin and areas of erythema and increased gingival volume of fibrous consistency with areas of necrosis. (1) Ulcers with irregular and raised borders on hard palate and gingival margin. (D) Ulcers with irregular and raised borders on the right cheek mucosa.
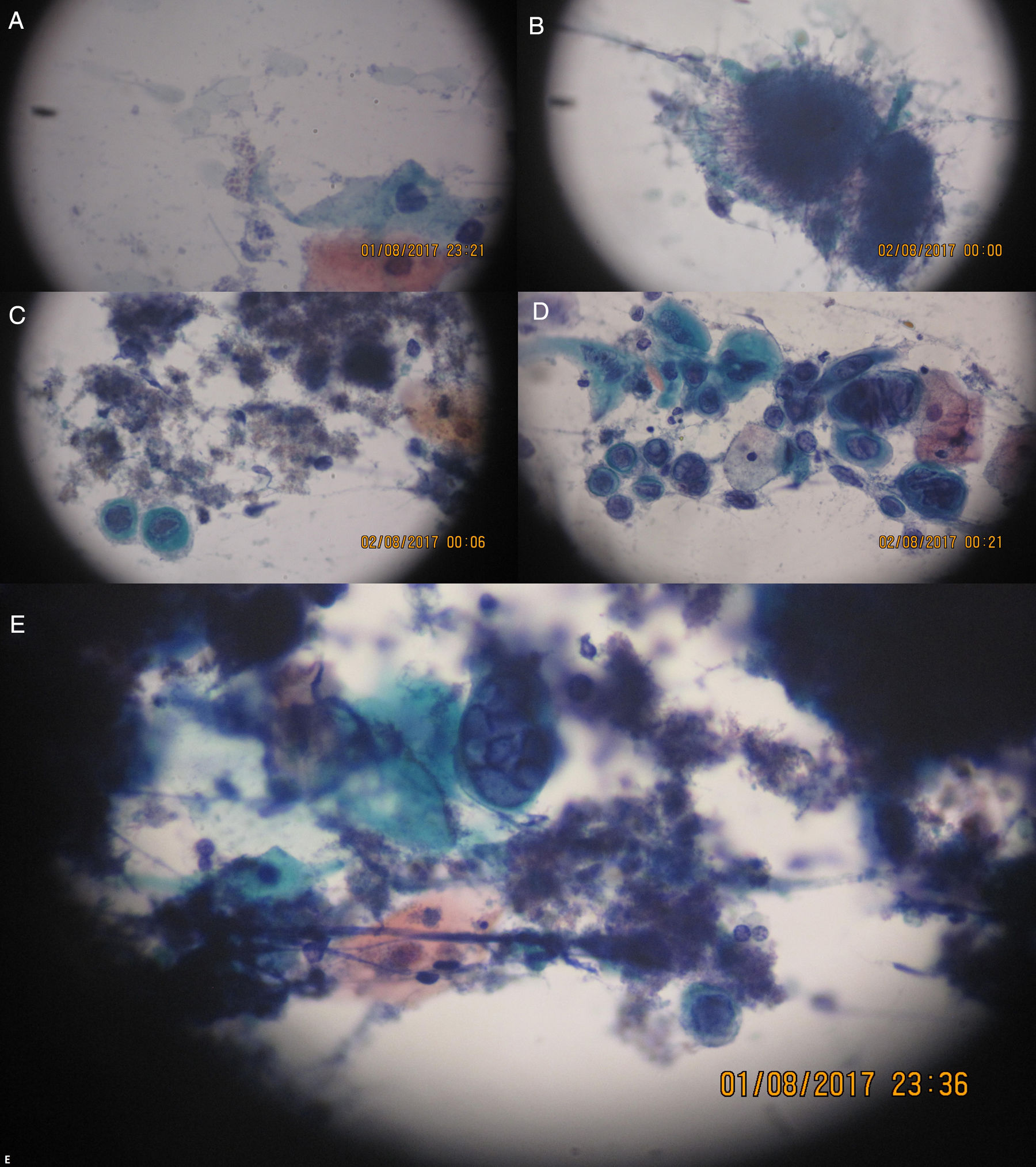
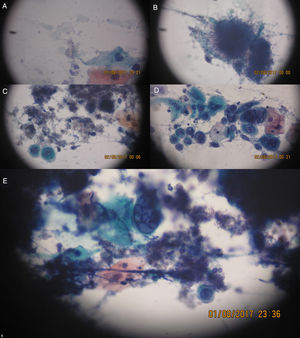
On this date, the patient's complete blood count was as follows: red cell count, 2.80 million cells/μL; global leukometry, 6300cells/μL; neutrophil count, 4366cells/μL; lymphocyte count, 1780cells/μL, and; platelet count, 12,000cells/μL. As the patient presented with anemia and thrombocytopenia, the decision was not to perform an incisional biopsy of the lesions. Instead, a scraping was taken from the right cheek mucosa, hard palate and upper right alveolar ridge for cytology specimen analysis. On the same date, the patient was started on acyclovir 400mg, taken orally every 4h, and 0.12% chlorhexidine mouthwash, used every 12h. On the next day (10/7/2016), the cytology report was as follows: smears showing squamous cells, often with multinucleation, nuclear molding, ground-glass chromatin, and some intranuclear inclusions, alongside intensely basophilic structures of filamentous appearance, suggestive of Herpes virus infection associated with the presence of Actinomyces and Candida (Figure 2).
(A) Spores and small fungal pseudohyphae. (B) Basophilic colony (very dark and purple) of filamentous appearance (looks like hairs). (C) Actinomyces filamentous colonies and left, below, a cell exhibiting intranuclear viral inclusion. (D) Several cells with viral cytopathic effect isolated, some binucleate or multinucleated, and chromatin with frosted glass image. (E) To the center, multinucleated cell with cytopathic effect by Herpes virus and around basophilic colonies of Actinomyces. The objective 20× and 40× were used with Papanicolaou stain.
Despite the stable leukometry, the patient was hospitalized after 3 days (10/10/2016), due to immunosuppression (IgA<50.0mg/dL and IgG=603mg/dL) to optimize the systemic therapy. At that time, the patient presented negative CMV antigenemia, positive CMV viral panel (real-time PCR) <25copies/mL and negative viral panel for varicella-zoster virus (VZV). He was treated with ampicillin 2g, intravenously administered every 6h for 21 days, in combination with intravenous (IV) acyclovir 1g every 12h, IV voriconazole, 200mg every 12h for 21 days, and 0.12% chlorhexidine in the form of mouthwash every 12h for 21 days. Total regression of the lesions occurred on 10/20/2016 and the patient was stable at the end of the treatment. The patient was discharged after 21 days and continued in outpatient treatment for an additional 28 days with amoxicillin, 1g every 8h. The patient continued in outpatient follow-up performed by the dental team. The case report was approved by the institutional ethics committee under number MS-60/2012, in accordance with the guidelines of Good Clinical Practice and the Brazilian law, and all patients signed an informed consent form.
DiscussionChronic GVHD is closely associated with oral infections and may cause dysfunction, as well as signs and symptoms that are detrimental to the quality of life.5,6 A diagnosis of chronic GVHD should be well established, avoiding misdiagnosis of other secondary infections, as bacterial infections progress more rapidly in immunosuppressed patients than in immunocompetent patients.5,6
According to Ochs et al.,5 bacterial infections are the most common of late-onset infections following HSCT, affecting 52% of cases and of these, about 51.5% are caused by Gram-positive bacteria.5 Viral infections accounted for 37% of the infections, the most common being Herpes simplex virus (15% involvement), and fungal infections were involved in 11% of cases, with candidiasis causing 33.3% of these infections.5
Regarding our case, the first diagnostic impression was Herpes virus infection due to the presence of ulcers inside the oral cavity, in keratinized areas.1 In immunocompetent patients, acute herpetic gingivostomatitis affects the intraoral region when it is primary, whereas secondary infection usually affects the lips. The disruption of the mucosal barrier by herpetic infection could have facilitated the infection by opportunistic microorganisms Due to the considerable increase in gingival volume and the dark red coloration with areas of necrosis, our diagnostic hypothesis was the existence of another infection associated with the herpetic infection, which led to the need for supplementary tests to reach a full diagnosis.
Based on the cytology results, the patient was diagnosed with HSV, Candida spp and Actinomyces spp. Actinomyces is a predominantly anaerobic, Gram-positive filamentous bacterium that is part of the bacterial microbiota of the oral cavity and its increase is usually associated with poor oral hygiene (characterized by the presence of periodontal pockets and dental biofilm), high alcohol consumption, smoking, and lung conditions (such as emphysema, chronic bronchitis and post-tuberculosis infection sequelae).7,8 They can affect various sites, such as the cervicofacial region, oropharynx, genital tract and digestive system.7–9 In post-HSCT patients, Actinomyces infections are rare, and few cases have been described in the literature.7,10
Biopsies for the diagnosis of Actinomyces infection are indicated because of the low sensitivity of the culture analysis, but due to the high risk of excessive bleeding in this case, a biopsy could have been an unsafe intervention. In this case, the decision was to perform the cytology test, which proved sufficient to make the diagnosis.
Actinomyces are bacteria usually present in the oral mucosa and therefore should only be identified as infections if sulfur granules are present.8 Evidence of several filamentous colonies of Actinomyces with sulfur granules was found in the cytological smears. The colonies, in their arachnoid presentation, also provide clear evidence of infection by Actinomyces spp. This infection usually presents a good outcome with prolonged antibiotic therapy,10 but treatment type and duration should be established on an individual basis. The drug of first choice is penicillin, but other regimens may be used with erythromycin, tetracycline, doxycycline and clindamycin.10
Candida spp infection is one of the main fungal infections in post-HSCT1 patients. It is diagnosed when the presence of hyphae and pseudohyphae in the oral mucosa smear is identified and, in this case, voriconazole treatment was implemented to avoid candidemia.
ConclusionRoutine examination of the patient's oral cavity after HSCT is extremely important because these patients are more susceptible to infections, compared to immunocompetent patients. In some circumstances, beyond the clinical examination, it is necessary to perform supplementary tests, such as exfoliative cytology, that contribute to establishing the diagnosis. This case illustrates how a quick diagnosis and an adequate early therapeutic approach are important for the rapid recovery and reduced morbidity and mortality in a transplanted patient.
Conflicts of interestThe authors declare no conflicts of interest.