Diversity in Classical Hematology Research
More infoAcute myeloid leukemia (AML) is most commonly presented in older adults; however, it appears 10 years earlier in Latin American countries. Clinical evolution in older adults from this populations has not been characterized. We analyzed outcomes and survival predictors.
MethodsPatients ≥ 55 years old diagnosed with AML at a hematology referral center from 2005 to 2020 receiving intensive chemotherapy (IC), low-dose cytarabine (LDAC) and best supportive care (BSC) were included. Survival analysis included the Kaplan-Meier and Cox models and the cumulative incidence of relapse (CIR).
ResultsSeventy-five adults were included and the overall survival (OS) was 4.87, 1.67 and 1.16 months, using IC, LDAC and BSC, respectively. The IC led to a higher OS (p < 0.001) and was a protective factor for early death, at a cost of more days spent hospitalized and more non-fatal treatment complications; non-significant differences were found between the LDAC and BSC. Eight (10.7%) patients underwent hematopoietic cell transplantation, with a higher OS (p = 0.013). Twenty (26.7%) patients achieved complete remission; 12 (60%) relapsed with a 6-month CIR of 57.9% in those < 70 years old vs. 86.5% in those ≥ 70 years old, p = 0.034. Multivariate analysis showed the white blood cell count (WBC) and IC had a significant impact on the patient survival, whereas chronological age and the Charlson comorbidity index (CCI) did not.
ConclusionAML in low-middle income countries demands a different approach; the IC improves survival, even with a high incidence of relapse, and should be offered as first-line treatment. Eligibility criteria should include WBC and a multidimensional evaluation. The age per se and the CCI should not be exclusion criteria to consider IC.
The highest incidence of acute myeloid leukemia (AML) is reported in older adults. It represents the most common form of acute leukemia, with a median age at diagnosis of 68 years.1 Based on the World Bank, 7% of the population from low- and middle-income countries are now aged 65 years or over, with an expected growth of 50% by 2030, making the optimal care of older adults with AML an international concern.1 Disappointingly, the improvement in survival seen in younger patients has not been replicated in older adults. This finding is a consequence of a lack of clinical trials that include elderly patients, due to their comorbidities, poor performance status, lower tolerability to intensive chemotherapy, socioeconomic factors and high-risk disease characteristics, among other reasons.2 Importantly, the global population aged 60 years could expect to live another 20.5 years on average, thus being diagnosed with AML at 60 years or older means dying at least a decade too soon.1
There is no firmly established standard of care for AML in this age group; 50% of patients do not receive treatment and patients eligible for intensive chemotherapy (IC) present higher rates of relapse and lower rates of long-term survival.3 The inevitable aging of our population underscores the need for a specific approach and treatment goals that could yield better outcomes.
AML is a biologically heterogeneous disease that offers different responses with uniform treatments. However, older adults of low-income populations have only been analyzed in one study and no study has been reported in Latin America,4 where a younger age at diagnosis is well known. Disparities in the clinical course and therapy response remain largely uncharacterized.
We studied older adults diagnosed with AML at a reference center for open-population patients and documented their clinical and laboratory features, their response to different treatment lines, relapse and survival rates.
MethodsWe conducted an observational, longitudinal and retrospective study of all patients who fulfilled the clinical and laboratory criteria for the diagnosis of AML over fifteen years, from January 2005 to January 2020, registered at the Hematology Department at the Dr. José Eleuterio González University Hospital, School of Medicine, Universidad Autónoma de Nuevo León, Monterrey, México. The institution provides healthcare for low-income uninsured patients from the open population in Northeast Mexico and patients who are routinely referred from public primary and secondary care centers. The study protocol was approved by the Ethics and Research Committee of the institution and is in full compliance with the Declaration of Helsinki, as revised in 2013.
The age cutoff to define the elderly may vary; we used ≥ 55 years old, as previously adopted, considering the 10-year earlier peak in AML diagnosis reported in our population.1,5–7 In addition to a complete medical history and physical examination, the diagnosis was established by a complete blood count, peripheral blood smear, bone marrow (BM) aspirate examination and immunophenotyping.8 The immunophenotyping was performed by the multiparametric flow cytometry, using the FACScalibur equipment (Becton-Dickinson, San Jose, CA.). Due to financial restrictions in the health system, cytogenetic studies were available only in 13 patients.
Complete remission (CR) was defined by a neutrophil count ≥ 1.0 × 109/L and a platelet count > 100 × 109/L in the peripheral blood and morphologically, as < 5% blasts in the bone marrow aspirate, accompanied by normal hematopoiesis.8 Relapse was defined as the reemergence of the criteria mentioned above for BM (≥ 5% blasts), the reappearance of myeloblasts in the blood or the development of extramedullary disease.8 Event-free survival (EFS) was defined as the timeframe from the date of diagnosis to the date of the first event (relapse or death for any reason) or the last follow-up visit. The overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up.8
The Charlson comorbidity index (CCI) was calculated for each patient. Early mortality was defined as death within 60 days of diagnosis. A morphologic subtype was assigned, using the French-American-British (FAB) classification. For this study, all patients diagnosed with the AML subtype M3 were excluded.
The intensive chemotherapy (IC) treatment consisted of a standard protocol composed of three days of an anthracycline (mitoxantrone 10 mg/m2/day or adriamycin 40 mg/m2/day) and seven days of continuous intravenous cytarabine (100 - 200 mg/m2/day).8 Systemic intensification was administered after induction to remission and consisted of intravenous cytarabine at 2 g/m2, delivered by a 4-hour infusion, on days 1 to 3, with a previous dose of 100 mg of hydrocortisone. After recovery, additional cycles of intensification, as described, were administered. The non-intensive chemotherapy consisted of low-dose cytarabine (LDAC) (10-20 mg/m2/day for 10 days, subcutaneous), with or without valproic acid (6 mg/m2 single dose), and the best supportive care (BSC) included vinblastine (10 mg i.v.), associated with palliative care.8
Statistical analysisThe SPSS version 25 (IBM Corp, Armonk, NY) was used for data analysis. A descriptive analysis was performed to obtain the mean, median and range. Comparisons between groups at diagnosis used the χ2 statistics for categorical variables. The overall survival (OS) and event-free survival (EFS) were assessed using the Kaplan-Meier method, calculating the time, survival and standard errors with 95% confidence intervals (CIs) and comparing the groups, using the Log-Rank test. Hazard ratios (HR) were assessed using a standard uni- and multivariate Cox regression analysis for the OS and EFS. The logistic regression analysis was used for early death. A p-value < 0.05 was considered statistically significant; variables with a p-value < 0.1 in the univariate analysis were included in the multivariate analysis. Univariate survival analyses used cumulative incidence functions and the Gray's test for relapse (CIR), as the non-relapse mortality was treated as a competing event.
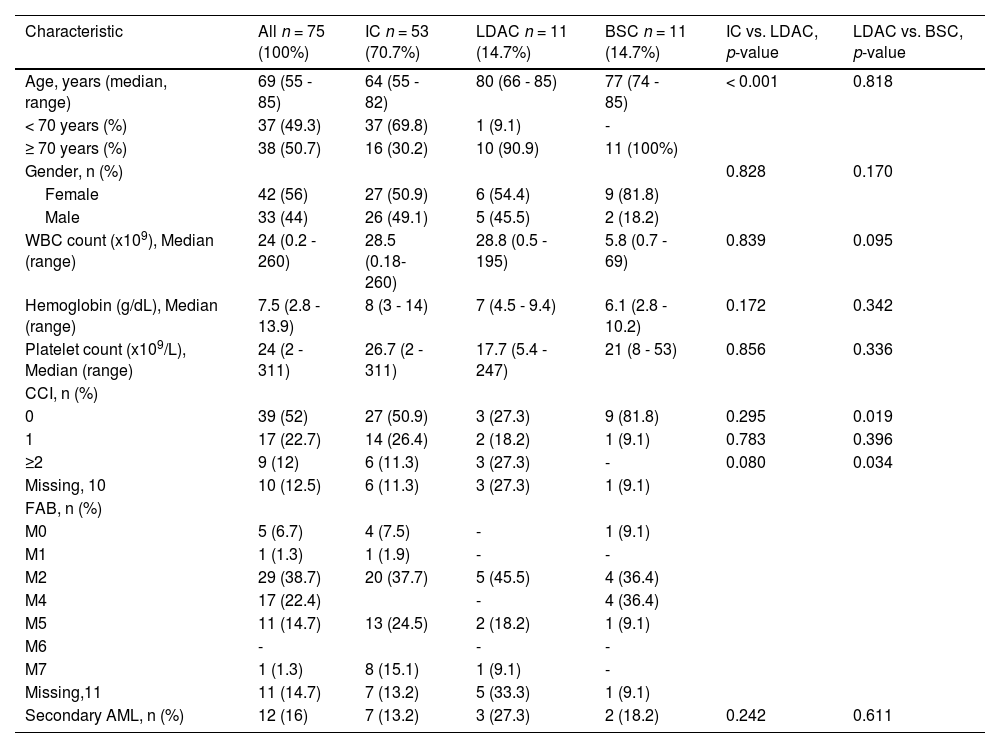
ResultsWe identified a total of 82 older adult patients with AML; seven were excluded due to incomplete clinical files. Of the remaining 75, 42 (56%) were females and 33 males (44%), with a male to female ratio of 1:1.3. The treatment consisted of an IC in 53 (70.7%), non-intensive chemotherapy with low-dose cytarabine (LDAC) in eleven (14.7%), and eleven (14.7%) received the best supportive care. The most frequent signs and symptoms were fatigue in 31.3%, weight loss in 13.8% and purpuric syndrome in 12.5%. Secondary AML occurred in twelve (16%) patients; eleven cases developed after the myelodysplastic syndrome (MDS) and one was associated with previous chemotherapy; no differences in the OS were observed between secondary and primary AML (p = 0.84). General characteristics and laboratory data of the three treatment groups are presented in Table 1. There were no statistical differences in these parameters, when comparing patients < 70 and ≥ 70 years old. Five patients (6.7%) declined treatment, 2 at the initial diagnosis and 3 after the IC; they received the BSC. There were no pertinent findings in risk assignment among the 13 patients who had cytogenetic studies.
Main clinical and laboratory data of 75 older adults diagnosed with acute myeloid leukemia in a low-income group in northeastern Mexico. Patients in the intensive chemotherapy group had a significantly lower age.
IC: intensive chemotherapy; LDAC: low-dose cytarabine; BSC: best supportive care; CCI: Charlson Comorbidity Index; FAB: French American British; WBC: White Blood Count.
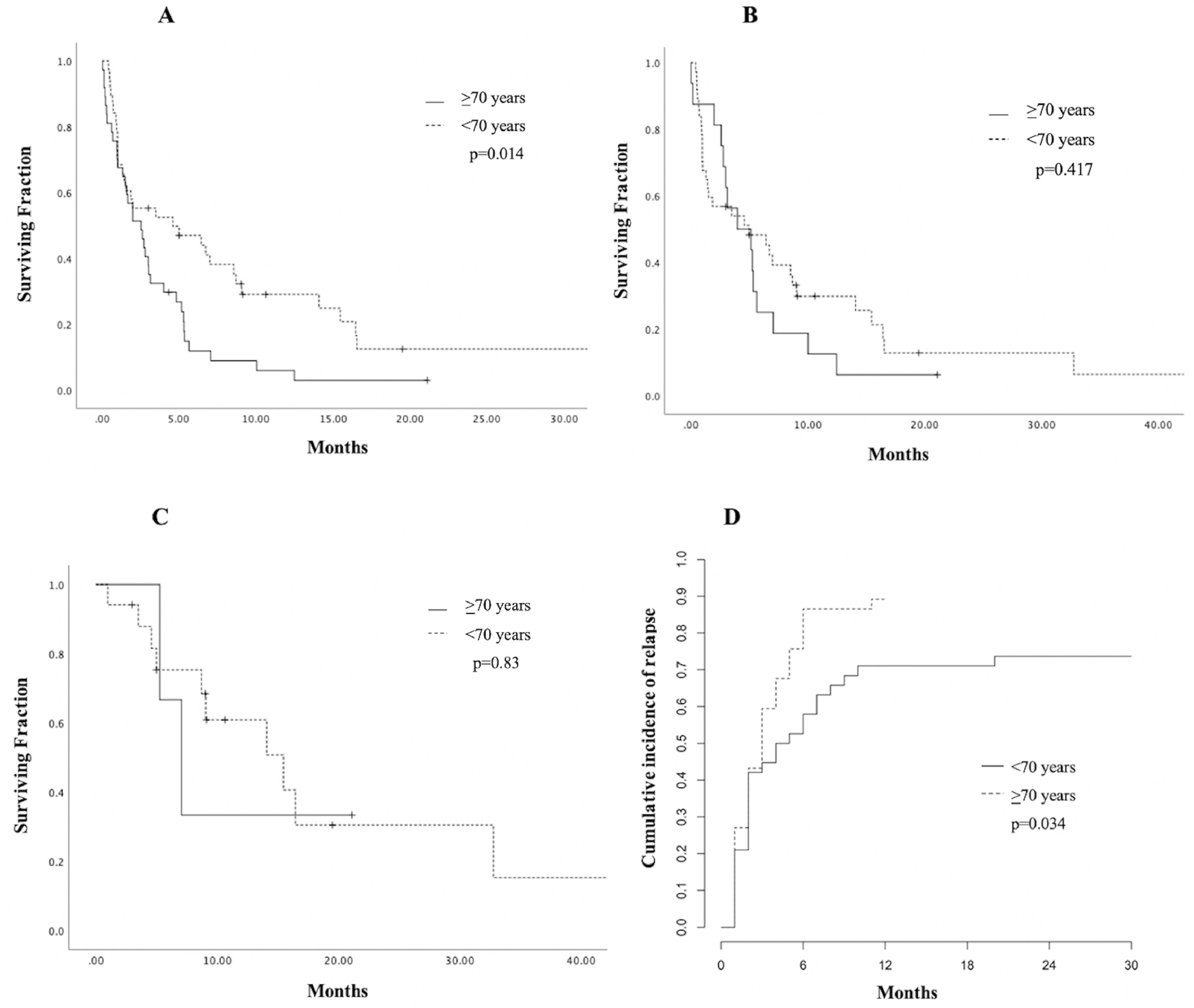
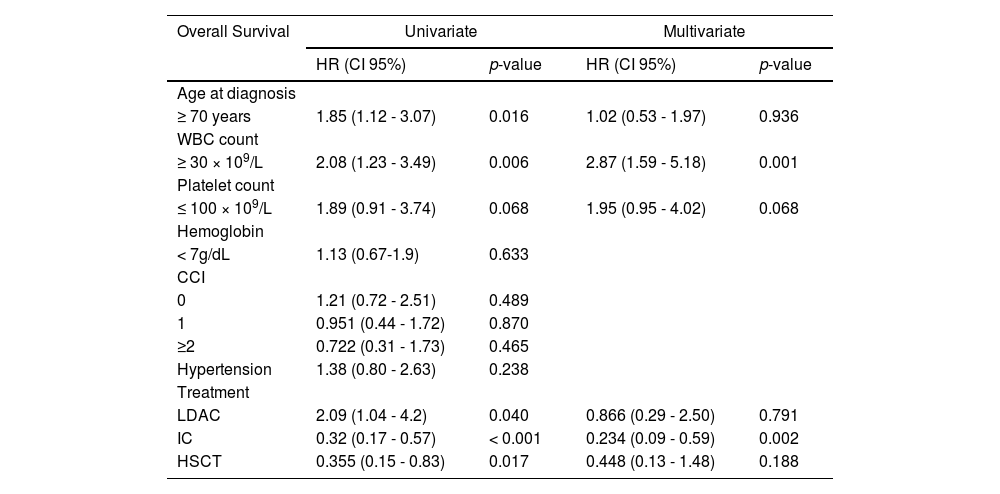
The CR was achieved by 20 patients (26.7%); 17 (85%) were < 70 years old and the remaining 3 (15%) ≥ 70 years old (p = 0.002) and all received IC. For this group achieving CR, the median OS was nine months (1 - 85), with a 1-year OS of 48% (CI 95%, 45.52 - 50.42) and a 3-year OS of 16% (CI 95%, 13.56 - 18.61), compared to 3.9% (CI 95%, 3.39 - 4.45) at one year among those who did not achieve CR (p < 0.001). During follow-up, 12 (60%) patients relapsed at a median time of 5.78 months (2 - 11); 7 (63.6%) were treated with second-line regimens mostly consisting of high-dose cytarabine, 4 (36.3%) received palliative care and 1 patient was lost to follow-up after relapse. No significant difference in OS was found in patients achieving CR, when comparing age groups (p = 0.83). Patients < 70 years had a significantly higher 3-year OS of 6.6% (CI 95% 1.12-3.07; p = 0.014, Figure 1, panel A). However, no significant difference in survival according to age was found in patients receiving IC (p = 0.417, Figure 1, panel B) or in those achieving CR (p = 0.83, Figure 1, panel C).The cumulative incidence of relapse at 6 months was 57.9% in patients < 70 years old (CI 95%, 56.2 - 59.4) and 86.5% for those > 70 years old (CI 95%, 85.2 - 87.6) p = 0.034, Figure 1, panel D.
Estimated survival and relapse incidence according to age in older adults with acute myeloid leukemia at a single center in northeastern Mexico. A. Overall survival (OS) plot according to age group. B. OS curve in patients receiving intensive chemotherapy (IC). C. Kaplan-Meier curve in patients achieving complete remission (CR). D. Cumulative incidence of relapse (CIR) in the cohort.
Complications in first-line treatment were found in 34 (45.3%) patients (97% were in the IC group and 3%, in the LDAC group, p < 0.001); the most common were febrile neutropenia in 18 (52.9%) and severe cytopenia in 10 (29.4%). Sixteen (20%) presented severe infection, of whom ten (62.5%), during induction therapy. The most frequently associated microorganisms were C. difficile, E. Coli and Aspergillum.
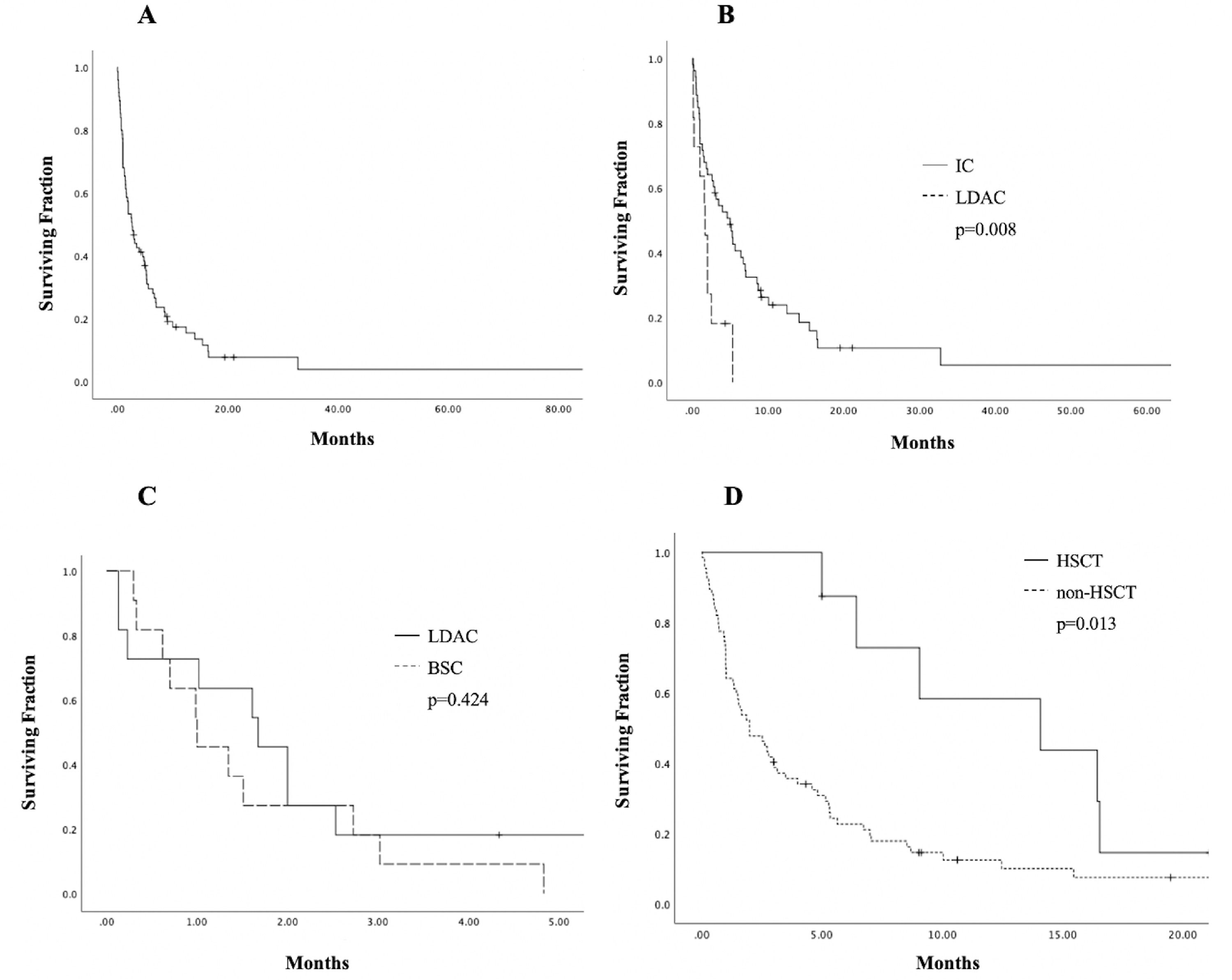
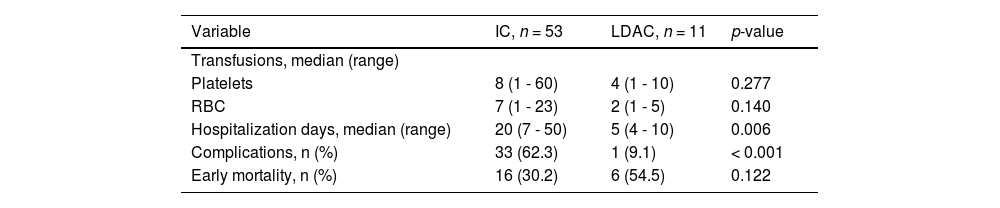
Eight patients (10.7%) underwent allogeneic hematopoietic stem cell transplantation (HSCT); 2 (25%) died early (< 30 days) after transplantation and 4 (50%), after relapse. The median OS for this group was fifteen months (6 - 21), with a 1-year OS of 43.8% (CI 95%, 40.08 - 47.44), compared to 10% (9.21 - 10.82) in patients who did not receive HSCT (p = 0.013), shown in Figure 2. This group of patients had a median age of 60 years (56 - 71), significantly lower than the 72 years in the rest of the cohort (p = 0.007).
Estimates of survival endpoints in 75 older adults with acute myeloid leukemia in a low-income group, according to treatment at an academic center in northeastern Mexico. A. Overall survival (OS) in the cohort. B. Kaplan-Meier curves for OS, comparing intensive chemotherapy (IC) and low-dose cytarabine (LDAC). C. Kaplan-Meier curves for OS, comparing LDAC and best supportive care (BSC) D. OS curves of patients receiving hematopoietic stem cell transplantation (HSCT).
In the last follow-up, 56 (74.7%) patients were dead; seventeen (30.3%) died during induction therapy, fourteen (25%), during treatment following induction, nine (16%), during relapse, two (3.5%), after HSCT, and the remaining fourteen (25%), after disease progression. The main causes of death were sepsis, followed by respiratory failure and severe bleeding.
The subgroup of seventeen patients (21.3%) who died during induction or post-induction treatment was further analyzed; results showed a median age of 65 (range 56 - 81), median WBC count of 39 × 109/L (range 0.18 - 244), CCI ≥ 2 in three (17.6%), median platelet count of 25 × 109 /L (5.2 - 13.9), median hemoglobin count of 8.3 g/dl (5.29 - 13.9) and a median of 7 platelet transfusion episodes (1 - 59) and 5 red blood cell (RBC) transfusions (1 - 8), with a median hospitalization of 10 days (4 - 30).
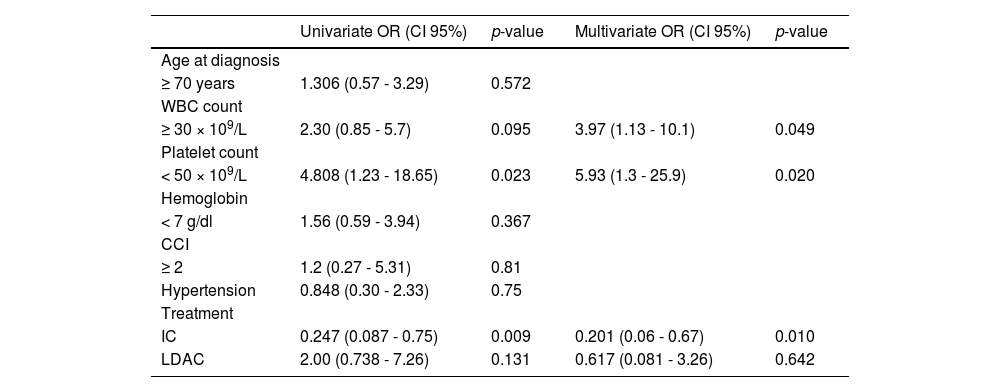
Early death occurred in 30 (37.5%) patients (IC 30.2%; LDAC 54.5% and BSC 72.7%; p = 0.166 in IC vs. LDAC and p = 0.375 LDAC vs. BSC). Most deaths were due to infections in 40.7%, progression, 26.6% or severe bleeding, 14.81%. In the multivariate analysis, a platelet count < 50 × 109/L and WBC ≥ 30 × 109/L at diagnosis were risk factors for early mortality (p = 0.020 and p = 0.049, respectively), whereas having received IC was a protective factor for early death (p = 0.010). Risk factors for early mortality are shown in Table 2.
Risk factors for early mortality in 75 older adults with acute myeloid leukemia in a low-income group at a referral center in northeastern Mexico. A WBC ≥ 30 × 109/L and a platelet count < 50 × 109/L were identified as risk factors, whereas intensive chemotherapy (IC) was a protective factor in the multivariate analysis.
WBC: White Blood Count; CCI: Charlson Comorbidity Index; IC: intensive chemotherapy; LDAC: low-dose cytarabine.
The median follow-up was 4.8 months (0.3 - 85.4), with a median OS and EFS of 2.73 and 2.41 months (0.3 - 85.4), respectively, and a 1-year OS of 15.4% (95% CI, 14.52 - 16.29) and 3-year OS of 3.9% (95% CI 3.30-4.56). The OS in the whole cohort is shown in Figure 2. Patients treated with IC presented a significantly higher median OS of 4.87 months (0.3 - 85) and a 3-year OS of 5.3% (95% CI 4.48-6.20; p < 0.001), whereas the LDAC group had a median OS of 1.67 months (0.13 - 5.32) and patients receiving BSC, 1.16 months (0.3 - 4.83). A significant difference in the OS was found between IC and LDAC (p = 0.008); in contrast, no significant difference was found in the OS, when comparing the LDAC and BSC (p = 0.42), Figure 2. In the univariate analysis, age ≥ 70 years (95% CI, 1.12-3.07, HR 1.85; p = 0.016), WBC ≥ 30 × 109/L at diagnosis (95% CI, 1.23-3.49, HR 2.08; p = 0.006) and treatment modalities impacted significantly on the OS. However, in the multivariate analysis, only a WBC ≥ 30 × 109/L (95% CI, 1.59-5.18, HR 2.87; p = 0.001) and having received IC (95% CI, 0.09-0.59, HR 0.234; p = 0.002) remained statistically significant; additional data are presented in Table 3.
Prognosis factors for overall survival (OS) in older adults with acute myeloid leukemia treated at a reference center. A WBC count ≥ 30 × 109/L and intensive chemotherapy (IC) remained significant risk and protective factors, respectively, for OS in the multivariate analysis.
WBC: White Blood Cell count; CCI: Charlson Comorbidity Index; LDAC: low-dose cytarabine;
IC: intensive chemotherapy; HSCT: Hematopoietic Stem Cell Transplantation.
A comparison of clinical characteristics and evolution between the IC and LDAC groups is shown in Table 4; significant differences were found in age (64 vs. 80 years, p < 0.001), median days spent hospitalized (20 vs. 5, p = 0.006), and treatment complications (62.3% vs. 9.1%, p < 0.001). No significant differences were found regarding these parameters, when comparing the LDAC to the BSC.
Outcomes and treatment complications in 75 older adults with acute myeloid leukemia receiving intensive chemotherapy (IC) and low-dose cytarabine (LDAC) strategies. Patients in the IC group had significantly more non-fatal complications and days spent hospitalized.
IC: intensive chemotherapy; LDAC: low-dose cytarabine, RBC: red blood cells; CCI: Charlson Comorbidity Index.
We report findings in a cohort of unselected, consecutive older adults diagnosed with AML over the last fifteen years at a hematology referral center for the open population in northeastern Mexico.
The AML prevalence in our population was lower than that reported in European cohorts.9,10 This could be explained by the ten-year earlier peak in diagnosis reported in Latin American countries, compared to the United States or the United Kingdom.11-15
The decision to treat seemed to be mainly influenced by age, as the IC group included significantly “younger” patients than the two other groups, as reported in previous studies.9,10 This variable has been consistently identified as the main patient-specific clinical factor for worse survival1,2,16; however, in our multivariate analysis, age was not an independent prognostic factor for early mortality or OS, in agreement with recent reports.17 The WBC, cytogenetics and CCI have also been identified as factors influencing treatment decisions.10,18 Still, in the present study, there was no significant difference in the WBC count at diagnosis between groups and the CCI did not seem to be a good indicator for decision-making or mortality in our patients. To the best of our knowledge, this is the first study evaluating the CCI in elderly Latin Americans with AML, indicating that perhaps a different scale, such as the ECOG, combined with a thorough geriatric evaluation, could be more appropriate in this population.
The median OS was 2.73 months and 3.9% at three years, comparable to the 2 to 3 months reported for previous cohorts,19,20 but considerably lower than the 7 months, with a 3-year OS of 18%, in a recent study including hypomethylating agents,10 which have been recently associated with significantly better outcomes; however, their cost ($55,332 - $74,160 USD a year)21 prevents their use in patients in low-middle income countries. Substantial differences in outcome between contrasting health economies have been reported in younger populations.22 These disparities are accentuated in older adults, as seen in this report.
Despite the significant improvement in survival seen in patients receiving the IC, compared to the LDAC, differences in clinical evolution indicate a higher proportion of non-fatal treatment complications and hospitalization care for patients in the IC group, which translates to a higher burden for the patient and the health system.9 Nonetheless, it is noteworthy that the IC acted as a protective factor for early mortality; therefore, it is not justified to deny intensive treatment in this group, based on presumed toxicity or early death rates.23
Age should be cautiously taken into consideration, when selecting treatment options for older adults with AML. Poor results and prognosis in older patients are partially due to confounding factors, such as “younger” patients, who are more prone to receive better treatment strategies, such as the IC and HSCT, as demonstrated in this report, and the subsequent lack of older patient representation in these therapies. In our group, older age appeared to have a worse prognosis, but when comparing only patients receiving IC or achieving CR, the impact of age in survival disappeared. Nonetheless, age was found to play a conspicuous and significant role in the incidence of relapse, as shown in our results.
These factors should be thoroughly considered by the patient and the physician to make a reasonable decision regarding treatment, as a previous study reported that 67% of the patients perceived that treatment was their only option and that they had not been offered a choice.24 Setting treatment goals requires the use of clinically relevant endpoints that, beyond survival, incorporate quality of life, good overall mental health, physical condition and freedom from severe complications.
The main limitations in our study include its retrospective nature, a reduced sample size, inherent to the demographics of the disease in the region, the lack of data on risk stratification for most patients, including cytogenetics and molecular markers, and unaccounted functional and social factors, which have an indirect influence on survival.25
ConclusionsThere are several conclusions in our report. Older patients with AML should receive intensive therapies as first-line treatment, whenever eligible, and hypomethylating agents should be made available to this end. Age per se or the mere presence of comorbidities should not be considered absolute exclusion criteria for the IC in this population. Finally, the study of older adults with AML in low-middle income groups should be encouraged, as it is becoming an epidemiologic issue, due to the ongoing inversion of the population pyramid, especially in countries as the one in this report, where information is scarce and availability of novel agents remains challenging.
We thank Sergio Lozano-Rodriguez, MD, for his critical review of the manuscript.