Hairy cell leukemia is an uncommon, indolent B-cell lymphoproliferative disorder. Therapy with cladribine (2-chlorodeoxyadenosine) is able to induce complete remission (CR) in the majority of patients after a single course of treatment. We report the outcomes of patients treated at Aga Khan University Hospital, Karachi, Pakistan.
MethodsThis was a retrospective review. Medical records of patients were used to collect data.
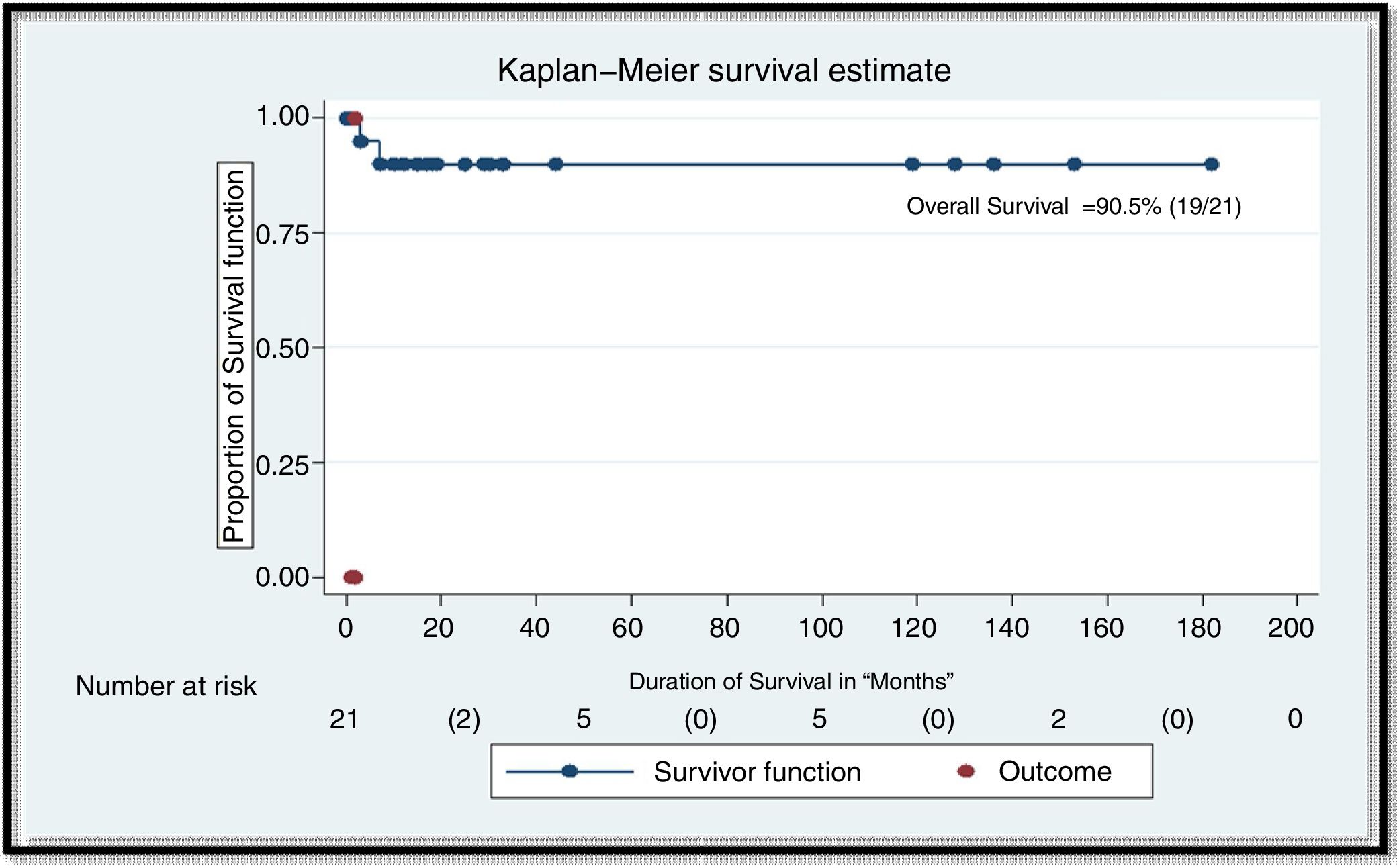
ResultsA total of 21 patients with hairy cell leukemia were treated with cladribine. All patients achieved an initial CR. Four patients (19%) required hospitalization and therapy for neutropenic fever. Six patients (29%) relapsed at a median of 48 months. All 6 patients were treated for relapse, out of which 4 achieved CR, 1 had partial response and 1 had refractory disease. The overall survival rate was 90.5%, with a median follow-up of 35 months.
ConclusionA single course of cladribine is able to induce CR in a vast majority of patients. Unfortunately, relapse is not uncommon. Patients who relapse can be successfully retreated with cladribine. Cladribine has impressive efficacy and a favorable acute and long-term toxicity profile when administered to patients with HCL.
Hairy cell leukemia is an uncommon, indolent B-cell lymphoproliferative disorder. Originally described by Bournocle et al.1 in 1958, it derives its name from the “hair-like” cytoplasmic projections emerging from its cell surface membrane. The median at age diagnosis for HCL ranges from 41 to 55 years (much younger than for other B-cell malignancies, such as chronic lymphocytic leukemia), with a predilection for the male gender (male to female ratio 4:1).2,3 HCL usually presents with splenomegaly with varying degrees of cytopenia(s) and diffuse bone marrow involvement by abnormal lymphocytes expressing CD11c, CD25, CD103, pan B-cell antigens (CD19, CD20 and/or CD22) and tartrate-resistant acid phosphatase.4,5
Although indolent, HCL is considered incurable. The therapeutic strategy for HCL has evolved over decades. Historically, not all newly diagnosed patients need treatment upfront.6 For those requiring treatment (usual indications for initiating treatment were cytopenias and/or symptomatic splenomegaly), splenectomy was the standard of care7 until 1984, when Quesada et al.8 explored interferon as treatment for HCL, though interferon had disappointing outcomes, with infrequent and short-lived complete responses (CR).9 Subsequently, nucleoside analogs, such as 2′-deoxycoformycin (pentostatin) and 2-chlorodeoxyadenosine (cladribine), emerged and are popular therapeutic options for HCL in current practice. In fact, monotherapy with a nucleoside analog is now the first-line therapy for HCL patients requiring treatment.7
Cladribine is a deoxyadenosine analog that resists deamination by adenosine deaminase, leading to intracellular accumulation of deoxygenated adenosine triphosphate, causing DNA strand breaks, disabling of DNA repair and eventual apoptosis, with lymphoid cells particularly vulnerable to its molecular effects.6,10 Although cladribine is not curative, it has demonstrated impressive results in HCL, achieving long-lasting CR in a majority of treatment-naïve patients and has shown remarkable activity in relapsed disease.5–7
Previously Bilwani et al.11 reported the outcomes of patients treated at Aga Khan University(AKU) from 1990 to 2003. Seven patients were treated, 6 of whom (85.7%) achieved CR, while one patient had refractory disease and died of sepsis. We report the outcome of 21 HCL patients diagnosed at AKUH and treated with cladribine from January 2006 to December 2016.
Patients and methodsWe conducted a descriptive, retrospective review of patient medical records at AKU, which is a 560 bed tertiary care center in Karachi, Pakistan. The Ethical Review Committee at AKUH reviewed and approved the study design, under the protocol number “3771-Pat-ERC-15”.
All cases of HCL were identified by the ICD-10 coding of medical records (ICD-10 code: C91.4). Only adult, treatment-naïve patient charts were reviewed. The diagnosis of HCL was confirmed by review of diagnostic bone marrow morphology, trephine biopsy and Immunophenotyping by flow cytometry.
Standard criteria were used to initiate treatment, define treatment response and re-treatment in patients with a diagnosis of HCL.12,13 Briefly, asymptomatic patients with cytopenia(s) (hemoglobin<10.0g/dL, platelet count<100×109/L and/or absolute neutrophil count <1.0×109/L) and patients symptomatic from cytopenias and/or splenomegaly were started on anti-HCL treatment.
CR was defined as the normalization of peripheral blood counts and resolution of splenomegaly. Partial response (PR) was defined as ≥50% improvement in the peripheral blood counts or splenomegaly compared to that at diagnosis. Relapse was defined by the development of new or progressive peripheral blood cytopenias, recurrence of splenomegaly or new clinical symptoms attributable to HCL.
Data were analyzed using the Statistical Package for Social Sciences 20 (SPSS Inc, Chicago, IL, USA). Categorical variables were reported as frequencies and percentage distributions, while means, standard deviations and medians were used to describe continuous variables.
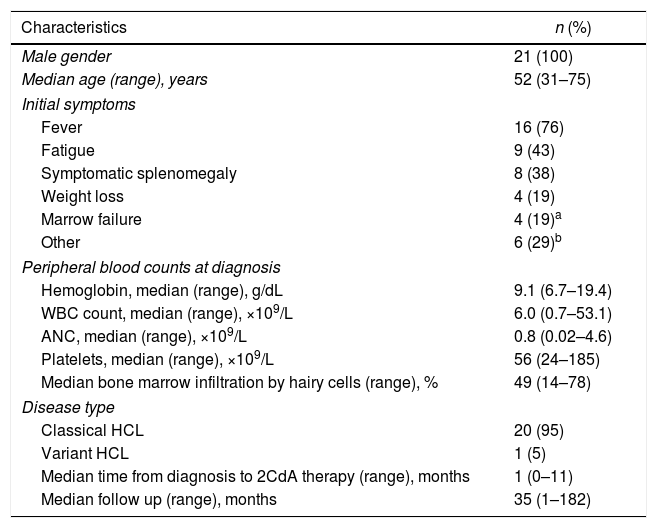
ResultsWe identified a total of 26 treatment-naïve patients who received treatment for HCL at AKU between January 2006 and December 2016. Twenty-one patients received treatment with cladribine and were included in this study. All patients were male, with the median age at diagnosis being 52 years (range 31–75). Fever was the most common presenting symptom (76% patients), followed by fatigue (43%) and symptomatic splenomegaly (38%). The median duration from HCL diagnosis to treatment with cladribine was 1 month (range 1–11). The median follow-up duration was 35 months (range 1–182). The baseline characteristics of these patients are shown in Table 1.
Baseline characteristics of 21 HCL patients treated with cladribine.
| Characteristics | n (%) |
|---|---|
| Male gender | 21 (100) |
| Median age (range), years | 52 (31–75) |
| Initial symptoms | |
| Fever | 16 (76) |
| Fatigue | 9 (43) |
| Symptomatic splenomegaly | 8 (38) |
| Weight loss | 4 (19) |
| Marrow failure | 4 (19)a |
| Other | 6 (29)b |
| Peripheral blood counts at diagnosis | |
| Hemoglobin, median (range), g/dL | 9.1 (6.7–19.4) |
| WBC count, median (range), ×109/L | 6.0 (0.7–53.1) |
| ANC, median (range), ×109/L | 0.8 (0.02–4.6) |
| Platelets, median (range), ×109/L | 56 (24–185) |
| Median bone marrow infiltration by hairy cells (range), % | 49 (14–78) |
| Disease type | |
| Classical HCL | 20 (95) |
| Variant HCL | 1 (5) |
| Median time from diagnosis to 2CdA therapy (range), months | 1 (0–11) |
| Median follow up (range), months | 35 (1–182) |
2CdA: cladribine; ANC: absolute neutrophil count; HCL: hairy cell leukemia; WBC: white blood cell.
Cladribine was given as a single continuous intravenous infusion. Eighteen patients (86%) received cladribine at a dose of 0.1mg/kg/day over the course of 7 days, 1 patient (4%), 0.09mg/kg/day over the course of 7 days, and 2 patients (10%), 0.14mg/kg/day over the course of 5 days. All 21 patients achieved a CR under cladribine therapy. During cladribine therapy, 4 patients (19%) required hospitalization and therapy with IV antimicrobials for neutropenic fever. Two patients had Staphylococcus aureus skins infections, 1 patient had Klebsiella pneumoniae pneumonia and 1 patient had Aspergillus fumigatus pneumonia. There were no deaths during or after initial therapy with cladribine in the 21 patients treated.
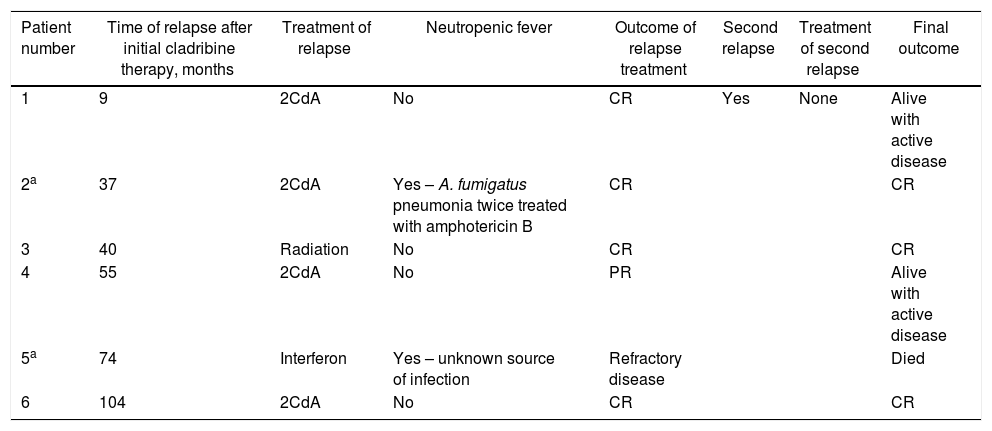
Six patients (29%) relapsed at a median of 48 months (range 9–104) after initial therapy with cladribine. The one patient with variant HCL did not experience relapse during the follow-up period. All six patients received treatment for relapse. Only one patient experienced a second relapse, but did not receive treatment. Two patients were admitted for neutropenic fever during therapy due to subsequent relapse (Table 2). One death occurred in the 6 patients receiving treatment for relapse due to septic shock. The treatment details and outcomes are elaborated in Table 2. All but 2 patients received cladribine at 0.1mg/kg/day over 7 days (similar to the initial therapy).
Characteristics of 7 patients who relapsed after initial therapy with cladribine.
| Patient number | Time of relapse after initial cladribine therapy, months | Treatment of relapse | Neutropenic fever | Outcome of relapse treatment | Second relapse | Treatment of second relapse | Final outcome |
|---|---|---|---|---|---|---|---|
| 1 | 9 | 2CdA | No | CR | Yes | None | Alive with active disease |
| 2a | 37 | 2CdA | Yes – A. fumigatus pneumonia twice treated with amphotericin B | CR | CR | ||
| 3 | 40 | Radiation | No | CR | CR | ||
| 4 | 55 | 2CdA | No | PR | Alive with active disease | ||
| 5a | 74 | Interferon | Yes – unknown source of infection | Refractory disease | Died | ||
| 6 | 104 | 2CdA | No | CR | CR |
2CdA: cladribine; CR: complete response; PR: partial response.
At a median follow-up of 35 months, 1 patient died. This patient had a relapse after initial therapy with cladribine (patient 5 in Table 2). His disease was refractory to therapy for relapse and he died of septic shock. The overall survival in our patient cohort was 90.5% (Figure 1).
Overall survival in n=21 patients. The overall response rate (ORR) was defined as the proportion of patients achieving a best clinical response. Overall survival rate (OS) was defined as time with beginning till death; patients still alive at the time of gathering the data were censored at the date of the last presented medical record. Descriptive analyses were applied to measure the specific treatment patterns and ORR. and overall survival rate were descriptively analyzed using Kaplan–Meier survival methods along with the log-rank test technique and Breslow test for statistical significance. Data was set at a 95% confidence interval at a 5% level of significance.
The present study elaborates the characteristics and outcomes of 21 HCL patients who were treated with cladribine at AKU between 2006 and 2016. The efficacy of cladribine in treating HCL was first reported by Piro et al.14 in 1990. Twelve patients were treated and 11 (92%) achieved CR with no relapses, at a median follow-up of 16 months. A study of 88 patients by Rosenberg et al.7 reported a CR of 88%. Other studies have also reported very impressive responses after treatment with cladribine, with overall response and CR rates ranging from 75 to 100% and 72 to 98%, respectively.3,5,6,15,16 The 100% CR rate in our cohort is in concordance with these previously reported figures. Unfortunately, relapse is not infrequent in the clinical course of patients, despite the excellent response to cladribine. Twenty-nine percent of the patients in our study suffered from disease relapse. Some studies have reported similar relapse rates (26–38%),3,5,17 while other studies have reported relatively higher percentages (58%).7 A recent study16 reported a surprisingly low (16%) relapse rate in HCL patients treated with cladribine, although one must note that the median follow-up in this study was relatively shorter than the follow-up in studies reporting higher relapse rates, indicating that relapse tends to become evident later rather than sooner. This is likely due to the indolent nature of HCL, indicating that the malignant clone that survives initial therapy with cladribine takes time to achieve enough mass to produce an evident relapse.
A prior experience at our center highlighted a CR rate of 85.7% in 6 patients, with a relapse rate of 50%.11 Other centers in Pakistan have also reported outcomes in HCL treated with cladribine. Buttar et al.,15 reported a CR rate of 82.3% in 17 patients treated with cladribine in their cohort of 22 HCL patients, with a surprisingly low (5.8%) relapse rate. Jameel et al.18 reported CR with no relapse in 2 patients treated with cladribine in their cohort of 7 HCL patients.
The most notable toxicity associated with cladribine therapy is myelosuppression leading to susceptibility to infectious complications. The incidence of neutropenic fever during first-line cladribine therapy in our cohort was 19%, which is lower in comparison to the reported 26–50% incidence in prior studies.4,6,19 Different dosing routes and schedules of cladribine have been studied in an attempt to modify the incidence and severity of neutropenia and treatment-related toxicity; however, these have shown comparable outcomes in terms of treatment response and toxicity.6 While one would think that adjunct use of filgrastim may help minimize the incidence of neutropenic fever, a phase II study20 showed no clinical benefit in terms of incidence of neutropenic fever and the need for hospitalization or longer hospitalization duration, despite an increased absolute neutrophil count (ANC) nadir and decreased time to ANC recovery after cladribine therapy.
In the case of relapsed disease (specifically for relapses occurring between 2 and 5 years after initial therapy), chemoimmunotherapy with rituximab and cladribine is an effective option,21 with the rituximab and cladribine combination yielding very impressive CR rates (with longer lasting CR in comparison to prior cladribine monotherapy) and failure-free and overall survival.22 Unfortunately, we are situated in a developing country and due to its heavy costs and limited availability,23 rituximab could not be used in any of the patients in our cohort.
To conclude, cladribine demonstrates highly impressive activity against HCL. Although our study shows a remarkable 100% CR rate to first-line cladribine therapy with a relapse rate of just 29%, our findings are limited by a small number of patients in our cohort and with a short median follow-up period in comparison to other published studies. It is possible that the relapse rate in our study is underestimated by the short follow-up duration. This warrants larger studies with extensive follow-up periods in treated patients to ascertain how effective cladribine is in HCL, not just upfront in inducing CR, but also in how long the CR lasts. Multicenter collaborations and prospective studies are the best way of achieving this goal and providing insight into maximizing the benefit derived from cladribine therapy.
Conflicts of interestThe authors declare no conflicts of interest.