To describe the oral health status of patients with multiple myeloma and compare to a control group.
Materials and methodsThe medical history of the studied subjects was obtained from the medical records and through interviews. Trained examiners evaluated the oral mucosa, teeth, periodontium and imaging aspects. The dental status was evaluated by the decayed, missing and filled teeth index. The presence of bone lesions was investigated with cone beam computer tomography images of the jaws.
ResultsThe most common oral mucosa features were paleness (31%) and coated tongue (14.3%) in the multiple myeloma group (N=42); and coated (21.4%) and fissured tongue (10.7%) in the control group (N=28). The mean DMFT index of patients with multiple myeloma was high, but not significantly different from controls (14.57 versus 19.69, p=0.975). Hypodense lesions suggestive of multiple myeloma were observed in the jaws of 73.8% of the patients. Hypodense lesions related to teeth were detected in 33.3% of the patients and in 53.6% of the controls (p=0.832).
ConclusionsThe studied population of multiple myeloma patients presented many oral health issues that needed attention. Thus, oral care should be included in the routine treatment to improve the quality of the oral status in these patients.
Multiple myeloma (MM) is a hematologic neoplasm characterized by a monoclonal plasma cell proliferation that usually presents the following clinical features: hypercalcemia, renal failure, anemia and bone lesions (CRAB).1 Some oral features may be observed in patients presenting MM, such as swelling, pain, gingival bleeding, paresthesia and osteolytic lesions.2–6 However, epidemiological data on the oral features are scarce in the literature and restricted to case reports or case series.2–5,7
Few studies have addressed the oral manifestations of MM. A literature review reported 14.1% (n=783) of the patients with MM presenting some type of oral feature.3 A retrospective study on 77 patients reported jawbone lesions occurring in 15.6% and oral mucosa lesions affecting 2.6% of them.8
The prevalence of caries and the periodontal condition in patients with MM have not been reported in full articles in the English language. These topics need to be addressed because both chronic dental disease and poor oral hygiene may develop into acute odontogenic infections and potentially life-threatening systemic conditions in immunosuppressed patients.9
Most of the patients with MM are on bisphosphonate therapy to minimize skeleton-related events, such as pain and bone fractures.10 One of the adverse reactions of these drugs is the medication-related osteonecrosis of the jaw (MRONJ).10 The MM patients on bisphosphonate therapy need accurate oral care in order to avoid invasive dental treatments and thus reduce the risk for the development of MRONJ.10
Soft and hard oral tissues may be affected in MM patients, either as a result of the disease, or related to its treatment. The aim of this cross-sectional study was to evaluate the oral health status, regarding soft and hard tissues of patients with MM, comparing them to controls.
MethodsStudy populationThis is a cross-sectional study which evaluated a convenience sample of patients with MM attended to at the Clementino Fraga Filho University Hospital (HUCFF) Hematology Clinic at the Universidade Federal do Rio de Janeiro (UFRJ). The study was approved by the institutional ethical committee under no. 693.402 and all the study subjects signed a consent form. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Out- and in-patients in treatment for MM who agreed to participate in the study were included. Patients with other gammopathies or hematological conditions, as well as those institutionalized for hematopoietic stem cells transplantation, were excluded from the study. Sociodemographic, oral mucosa, dental and bone imaging characteristics were compared to those of a control group without MM. The patients with MM were not on active dental treatment upon investigation. They were all referred for oral care at the HUCFF/UFRJ Oral Health Program clinic. Controls were recruited from the individuals with appointments for cone beam computed tomography (CBCT) imaging of the jaws at the UFRJ Dental School Oral Radiology Clinic during the study period. Controls were gender and age-matched candidates for rehabilitation with dental implants. They were excluded if conditions affecting bone remodeling (such as bone metastasis, thyroid surgery, radiotherapy and metabolic bone diseases) were reported, or if they had history of bisphosphonate therapy.
Data collectionSociodemographic and clinical characteristics from both groups were collected through interviews. The clinical data on MM patients were also collected from medical records. Oral exams and CBCT imaging were performed for all subjects.
The oral exams were performed under a light-emitting diode head lamp, using wooden tongue depressors, and a dental mirror. Oral exams of MM patients were performed in a hospital chair at the Hematology Clinic and the exams of individuals in the control group were performed at the Oral Radiology clinic.
Extra- and intraoral exams were performed under standard guidelines.11 If oral lesions were observed, the clinical characteristics of the lesions were recorded and the lesions were photographed. An initial diagnosis was provided by two trained examiners (E.F.F. and C.A.B.). After the initial diagnosis, an experienced stomatologist (S.R.T.) confirmed the diagnosis by reviewing the photographs. Cases that needed further investigation or monitoring were referred to the HUCFF/UFRJ Oral Health Program clinic.
The decayed, missing and filled teeth index (DMFT) was used to measure the caries history of all the subjects. The exam was conducted by a single calibrated dentist (E.F.F.), following the calibration protocol of the World Health Organization (WHO)12 (intra-rater kappa=0.74 [95% CI: 0.62–0.89]). The use of a partial or complete removable dental prosthesis was recorded for both arches. The need for prosthetic rehabilitation was also registered.
All CBCT imaging exams were conducted by one experienced oral radiologist (F.R.G.). All CBCT procedures were standardized and obtained with the Kodak K9500® (Carestream Health, Rochester, NY, USA) equipment, stored and later analyzed by a single dentist (E.F.F.), using the CS 3D software (version 3.1.9; Carestream Health, Rochester, NY, USA). Bone lesions were recorded as related or not to teeth, when appropriate.
Data analysisThe statistical evaluation was performed using the SPSS 17.0 statistical package (SPSS Inc., Chicago, IL, USA). Descriptive data analyses were reported as the absolute frequency and percentage for categorical variables, as well as the mean and standard deviations for the continuous variables. The presence of soft tissue or tooth-related bone lesions and the use of dental prosthesis were compared between groups using the chi-square test. The DMFT index of patients with MM was compared with that of the control group using the Student's t-test. The kappa test was used to verify the reliability of the investigators during calibration and the correlation of bone lesions in the body and the jaws. The values of p<0.05 were considered statistically significant.
ResultsA total of 74 subjects were enrolled in the study (43 MM and 31 controls). Four were excluded for the following reasons: one MM patient refused to participate, and three controls were excluded for having a history of bisphosphonate therapy. Thus, forty-two patients were included in the MM group and 28 individuals, in the control group.
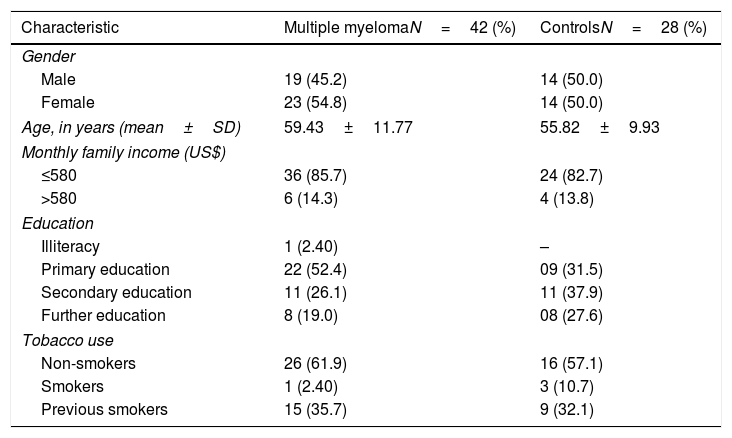
The sociodemographic characteristics from both groups are shown in Table 1. Individuals from both groups showed similar sociodemographic characteristics, for there were no statistical differences between groups. In the MM group, the most common medications were dexamethasone (60%), thalidomide (60%), melphalan (10%), prednisone (10%), thalidomide (10%), cyclophosphamide (4%). Thirty-three (78.6%) patients in the MM group were on endovenous bisphosphonate therapy.
Sociodemographic characteristics of the 70 subjects in the study.
| Characteristic | Multiple myelomaN=42 (%) | ControlsN=28 (%) |
|---|---|---|
| Gender | ||
| Male | 19 (45.2) | 14 (50.0) |
| Female | 23 (54.8) | 14 (50.0) |
| Age, in years (mean±SD) | 59.43±11.77 | 55.82±9.93 |
| Monthly family income (US$) | ||
| ≤580 | 36 (85.7) | 24 (82.7) |
| >580 | 6 (14.3) | 4 (13.8) |
| Education | ||
| Illiteracy | 1 (2.40) | – |
| Primary education | 22 (52.4) | 09 (31.5) |
| Secondary education | 11 (26.1) | 11 (37.9) |
| Further education | 8 (19.0) | 08 (27.6) |
| Tobacco use | ||
| Non-smokers | 26 (61.9) | 16 (57.1) |
| Smokers | 1 (2.40) | 3 (10.7) |
| Previous smokers | 15 (35.7) | 9 (32.1) |
SD: standard deviation; US$: United States dollars.
All p-values >0.05.
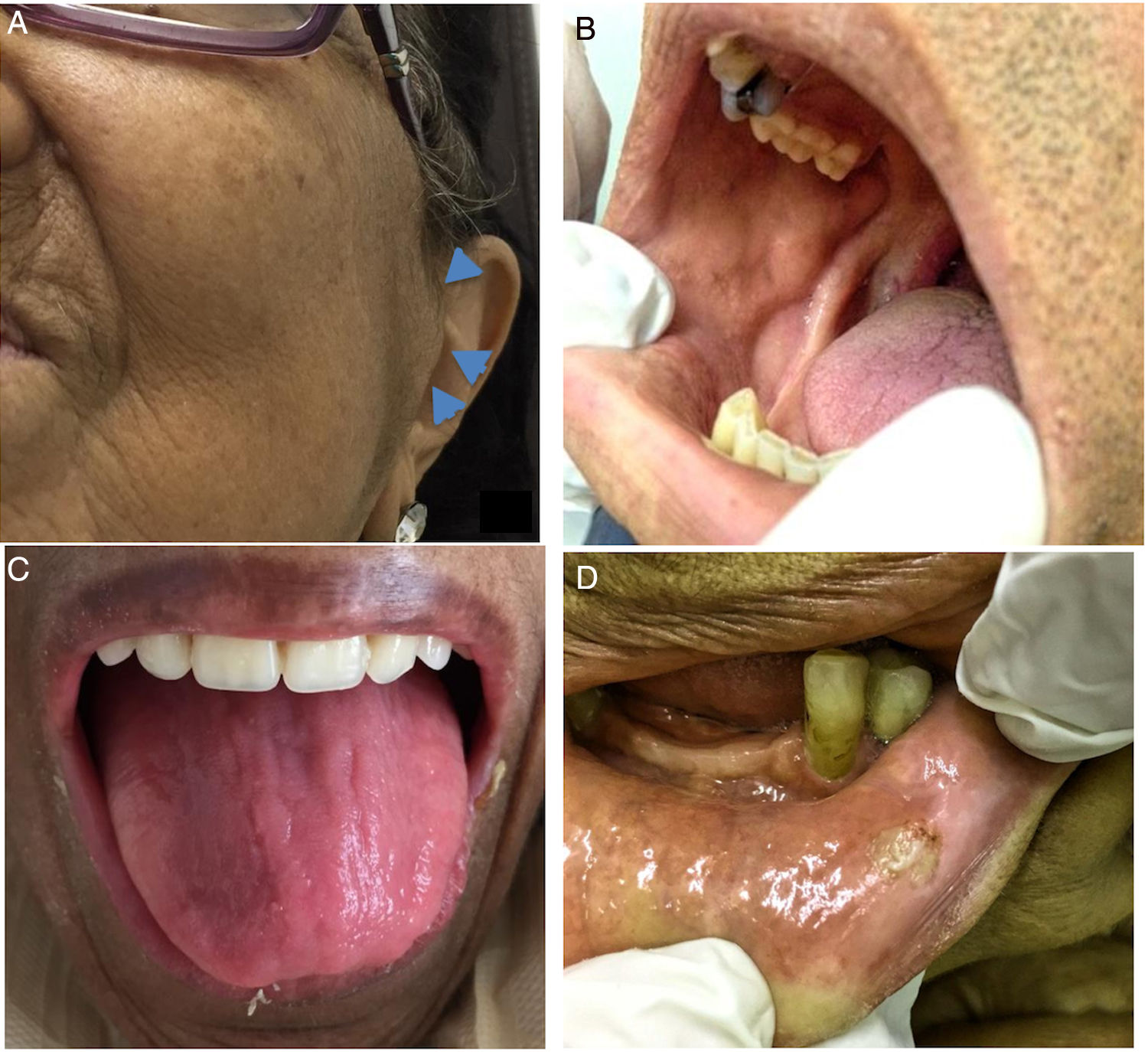
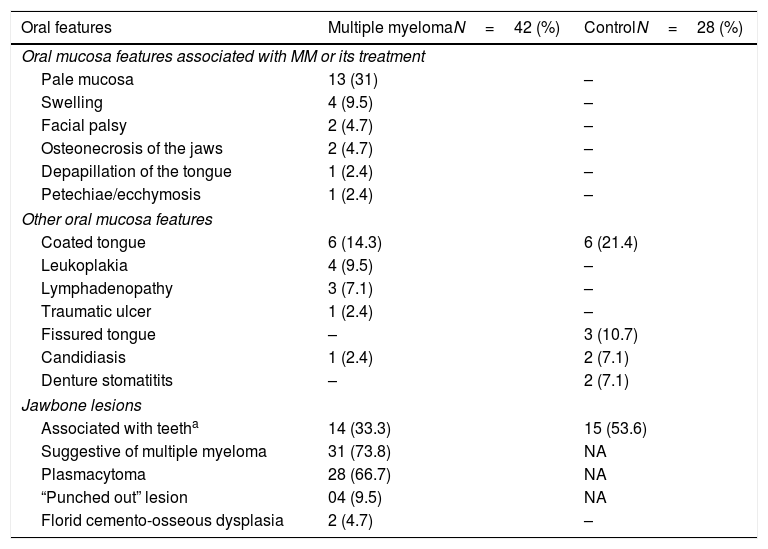
The extra-oral features observed in MM patients were lymphadenopathy (n=3/7.1%) and facial palsy (n=2/4.8%). Overall, the intra-oral soft tissue features were more frequently observed in patients with MM (n=30/71.4%) than in individuals from the control group (n=11/39.2%; p=0.049) (Table 2). In the MM group, the most prevalent soft tissue features were pale oral mucosa, followed by coated tongue, swelling and leukoplakia (Figure 1). In the control group, coated tongue was the most prevalent soft tissue feature. Among these oral features, there were 23 (54.7%) that were related to MM or its treatment.
Oral features observed in the 70 studied subjects.
| Oral features | Multiple myelomaN=42 (%) | ControlN=28 (%) |
|---|---|---|
| Oral mucosa features associated with MM or its treatment | ||
| Pale mucosa | 13 (31) | – |
| Swelling | 4 (9.5) | – |
| Facial palsy | 2 (4.7) | – |
| Osteonecrosis of the jaws | 2 (4.7) | – |
| Depapillation of the tongue | 1 (2.4) | – |
| Petechiae/ecchymosis | 1 (2.4) | – |
| Other oral mucosa features | ||
| Coated tongue | 6 (14.3) | 6 (21.4) |
| Leukoplakia | 4 (9.5) | – |
| Lymphadenopathy | 3 (7.1) | – |
| Traumatic ulcer | 1 (2.4) | – |
| Fissured tongue | – | 3 (10.7) |
| Candidiasis | 1 (2.4) | 2 (7.1) |
| Denture stomatitits | – | 2 (7.1) |
| Jawbone lesions | ||
| Associated with teetha | 14 (33.3) | 15 (53.6) |
| Suggestive of multiple myeloma | 31 (73.8) | NA |
| Plasmacytoma | 28 (66.7) | NA |
| “Punched out” lesion | 04 (9.5) | NA |
| Florid cemento-osseous dysplasia | 2 (4.7) | – |
MM: multiple myeloma; NA: not-applicable.
Some subjects presented more than one oral feature.
There was no statistical difference in the mean DMFT index between the groups (14.57 for the MM group versus 19.69 for the control group, p=0.975). When the caries history was analyzed separately, a higher mean of filled teeth was observed in the control group, when compared to that of the MM group (6.28±5.55 versus 3.35±4.14, p=0.014). No statistical differences were observed in the number of decayed teeth (2.35±3.92 MM group versus 2.57±3.15 control group, p=0.810) and missing teeth (14.28±9.05 MM group versus 12.25±8.58 control group, p=0.350) between the studied groups. There were 24 (57.1%) patients using dental prosthesis in the MM group and 18 (64.2%) in the control group (p=0.227). Thirty patients (71.4%) in the MM group and 18 (64.3%) among controls needed prosthetic rehabilitation (p=0.332).
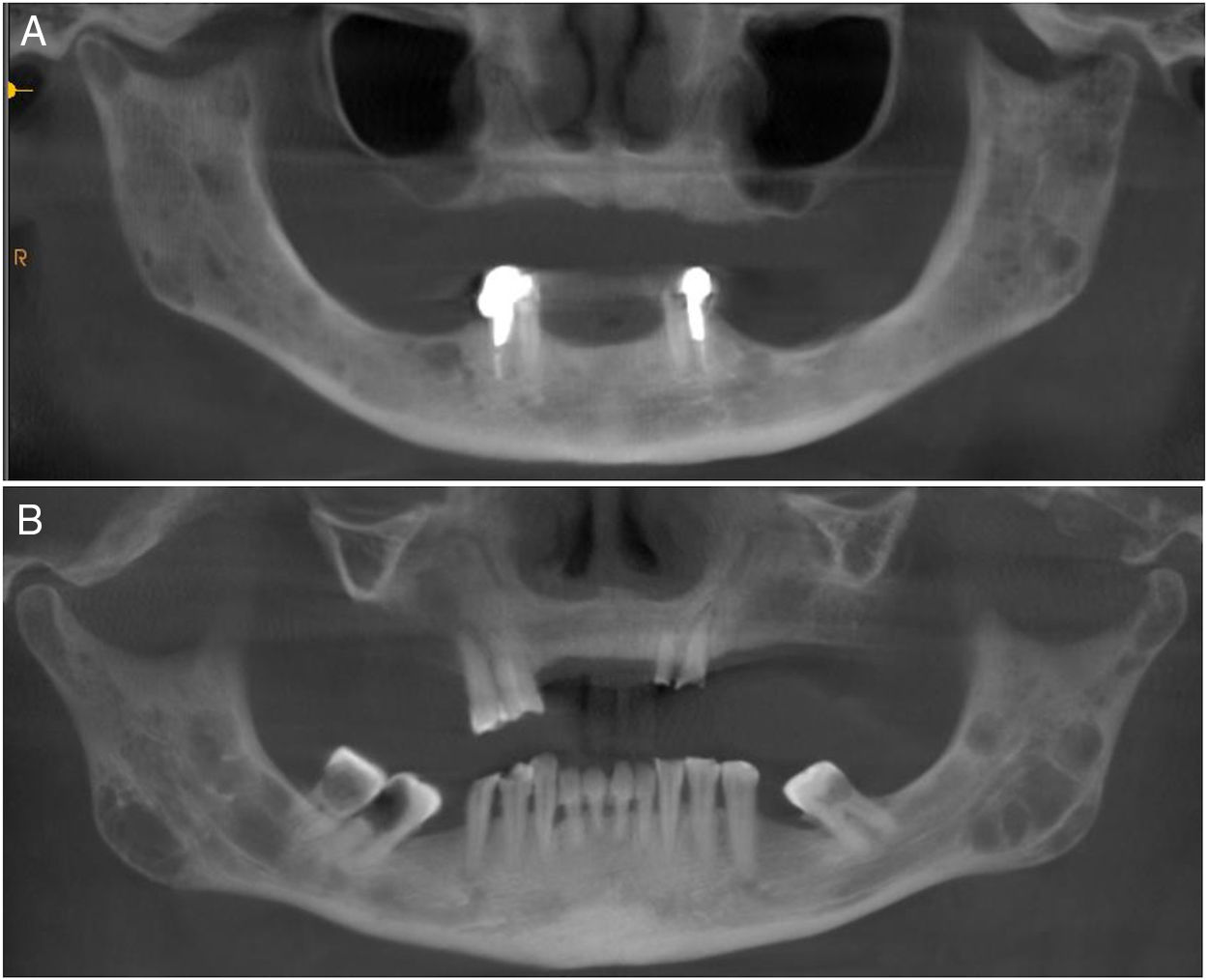
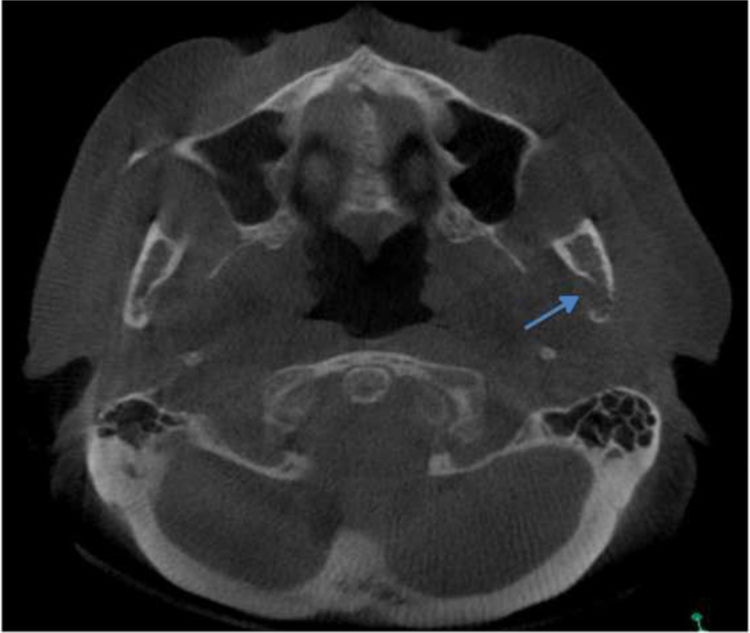
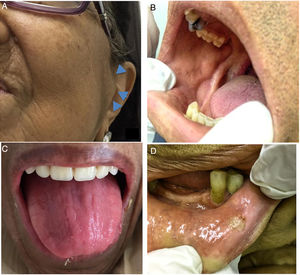
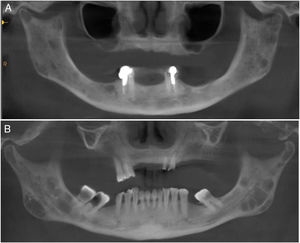
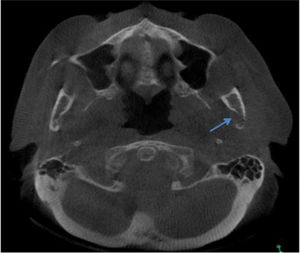
Regarding the imaging aspects of the jawbones in the MM group, solitary or multiple hypodense lesions suggestive of bone involvement were observed in 31 (73.8%) patients. In 28 (83.1%) patients, there were large lesions, with poorly defined margins, with a typical pattern of plasmacytoma. Minor multiple hypodense lesions, exhibiting sclerotic margins, named “punched out” lesions, were observed in the images of 5 (16.1%) patients (Figure 2). Cortical disruption was observed in 7 (22.5%) patients (4 in the upper jaw and 3 in the lower jaw) (Figure 3). The lower jaw was affected in 16 (51.6%) patients and both upper and lower jaws were affected in 15 (48.3%) cases.
Bone hypodense lesions associated with teeth were observed in 33.3% of the CBCT images of MM patients and in 53.6% of controls (p=0.832). Diffuse hyperdense lesions suggestive of florid cemento-osseous dysplasia were observed in 2 (6%) female patients with MM.
Data on the presence of bone lesions in the body skeleton were taken from the medical records. Thirty-four (81%) of the 42 MM patients presented at least one skeletal lesion in bones other than the jaws. A statistically significant correlation was found between the presence of the jawbone lesions suggestive of MM and the presence of bone lesions in other parts of the body (Kappa=0.75; p=0.026).
DiscussionIn the studied population of patients with MM, a high frequency of oral manifestations associated with the disease was observed in the soft (54.7%) and hard (78.5%) tissues. These data show a higher prevalence of oral features associated with MM than the data reported in other studies.3,8 Most of these manifestations are not pathognomonic of the disease, but may be associated with the condition or its treatment.
Jawbone lesions in MM are commonly mentioned and have been reported as one of the first detected signs of the disease.5,6,8,13–16 Similarly to the results of this study, plasmacytoma lesions6,8 have been described more often than the typical pattern of “punched out” lesions.3,17 Plasmacytoma lesions have been characterized as large and irregular radiolucent lesions, with poorly defined margins.6,8 “Punched out” lesions are commonly visualized as multiple and rounded radiolucencies with no corticalized well-defined margins.3,17 The jawbone lesions are not pathognomonic of MM. Differential diagnosis with other maxillary pathological conditions, such as chronic osteomyelitis, osteonecrosis, osteoradionecrosis, ameloblastoma, osteosarcoma, Paget's disease and other conditions, should be considered in the evaluation of the jaw images.
The majority of MM patients (73.8%) presented jawbone lesions suggestive of MM, as observed in the CBCT images. Other studies have reported smaller frequencies of jawbone lesions, varying from 5.18% to 15.60%.3,8,17 Most of the studies described the imaging aspects of MM mainly in panoramic radiographies,2,6,8,18 while others used intraoral radiographies,2,4,8 computed tomography5 and magnetic resonance imaging.2,5 The high number of jaw bone lesions identified in this study suggests that CBCT is more sensitive than other imaging methods in the detection of myelomatous lesions in the jawbones. The significant correlation between the presence of the bone lesions suggestive of MM in the jaws and other bones suggests that CBCT imaging is a powerful tool in the jaw evaluation of patients with MM. Because of a three-dimensional view of the jawbone, CBCT imaging may show more details, presenting good accuracy, without overlays, and deliver smaller radiation doses than conventional computed tomography. To our knowledge, only one study evaluated the maxillofacial complex of patients with MM using the CBCT as a tool to analyze possible lesions due to multiple myeloma, and found similar results.2
The mandible was affected in nearly half of the patients with MM presenting jaw lesions, and the other half presented simultaneously affected lower and upper jaws. No other study reported lesions in both upper and lower jaws at the same time. Some case reports report the mandible,6,17,18 while others report the maxilla,2,4,5,19 as the most affected gnathic bone in patients with MM.
Regarding oral soft tissue examination, many of the observed features were not MM disease-specific and may represent a response to local or systemic conditions. The prevalence of these features was higher than in the general population living in the same geographical area.20
The most common aspect observed in the soft tissue examination of MM patients was the paleness of the oral mucosa, and this aspect was not observed in the control group individuals. Pale oral mucosa has not been reported as an oral manifestation of MM. It is believed that this feature is probably a consequence of anemia,21 which is an indicator of disease progression.22 Paleness may be a subjective feature, and may be hard to be perceived by examiners.23 Interestingly, a significant association between pale oral mucosa and anemia has not been observed in the studied MM patients (p=0.558, data not shown). In the mouth, anemia may also lead to angular cheilitis or loss of the tongue papillae, which was observed in one of the patients in the study.21
Leucoplakia was observed in 9.5% of the patients with MM, but one cannot affirm that it is related to the disease. This condition may represent a clinical aspect of different histological degrees of dysplasia, and it is frequently associated with smoking habits,24 although none of these patients presented a smoking history.
Candidiasis was identified in only three patients among both groups, as shown in Table 2. This fungal infection may occur as a consequence of systemic conditions, medications or the use of dentures.25 Candidiasis was reported in only one MM case in the literature.26 Although there is no evidence that the Candida spp. infection is an oral manifestation of MM, it is known that patients with MM are more susceptible to infections, as a consequence of the therapy and severity of the disease.27
Coated tongue was the most prevalent oral feature in the control group, and was the second most frequent soft tissue feature in the mouth of patients with MM. This saburral aspect of the tongue may also be a result of lack of hygiene.21 Dentists should instruct patients on oral care to prevent this and other dental problems, such as caries disease.
Neurologic oral symptoms of MM are less common, with an estimated incidence of 1% of cases.28 In this study, the seventh cranial nerve was affected in two individuals, resulting in facial palsy. Some explanations for this aspect may be: direct manifestation in the course of the disease, infiltration of plasma cells in the central nervous system, adverse effects of some drugs used in the treatment, metabolic consequences of CRAB and immune effect of monoclonal proteins directed against different neural structures.28
Infections are considered an important cause of morbidity and mortality in patients with MM.29 Thus, lymphadenopathy is not rare in these patients. In this study, 7.1% of the patients presented lymphadenopathy in the cervical region. It has been reported that the estimated risk of infections in this population is seven times higher than that of healthy individuals.29
Other important observed data in this study were the precarious dental conditions with high frequencies of caries and need for prosthetic rehabilitation. Even though patients with MM presented high DMFT indexes, there were no significant differences between the MM group and controls. These findings may be explained by the low education level and low family income of the population. Special attention to the improvement of oral hygiene among patients with cancer may prevent complications during treatment.10,30 Good oral health could also contribute to the prevention of MRONJ,30 in individuals under bisphosphonate therapy. This is a morbid condition characterized by exposed bone in the mouth, which is mainly triggered by tooth extraction.10
Other oral manifestations like amyloidosis,16 trismus,6 root resorption,3 gingival hyperplasia,2,7 dental abscess3 and burning mouth syndrome,26 have been reported in patients with MM, but they were not observed in the patients of this study. The sample size in this study may not have allowed for the registration of all possible oral manifestations of MM. Furthermore, this epidemiological study describes the prevalence of the more common oral characteristics of MM, and the other studies were case reports with descriptions of incidental cases. Future studies are needed to define all the possible oral presentations of MM.
In conclusion, the studied population of MM patients presented high frequencies of oral features associated with disease in the jawbones and oral mucosa. The oral health condition of MM patients was poor, with a high frequency of caries and need for prosthetic rehabilitation. Oral care should be included in the routine treatment of patients with MM and dentists should be included in the multidisciplinary team of supportive care for patients with this condition.
FundingThis research was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ (JCNE E-26/103.046/2012), grants to Dr. Sandra R. Torres. The investigator Édila F. Feitosa is under the scholarship program (BN10 E-26/200.542/2018), from the same institution.
Conflicts of interestThe authors declare no conflicts of interest.