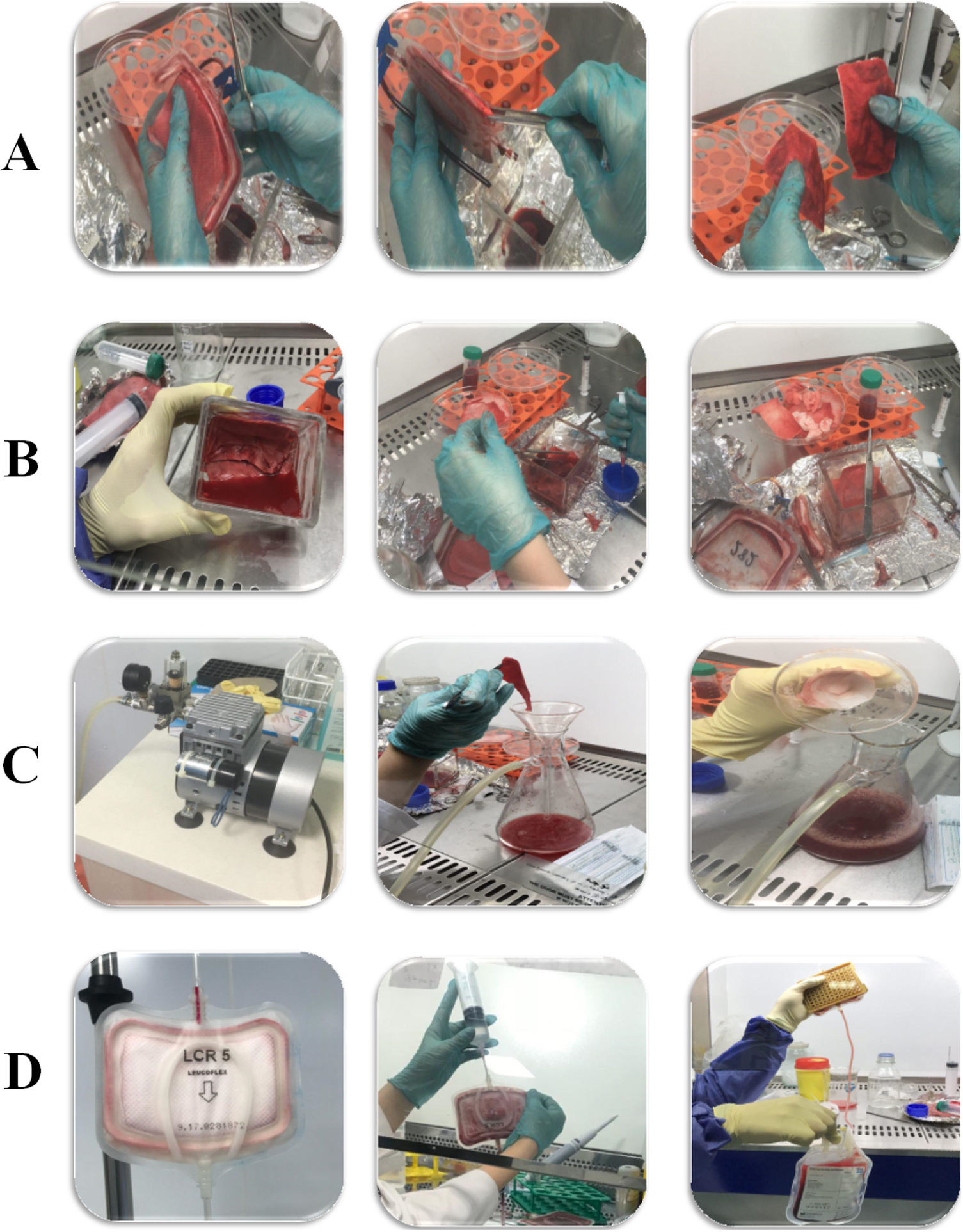
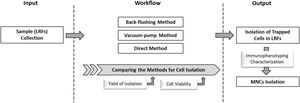
The isolation of captured peripheral blood mononuclear cells (PBMNCs) from leukoreduction filters (LRFs) can be of great importance in terms of bringing the lost cells back into use.
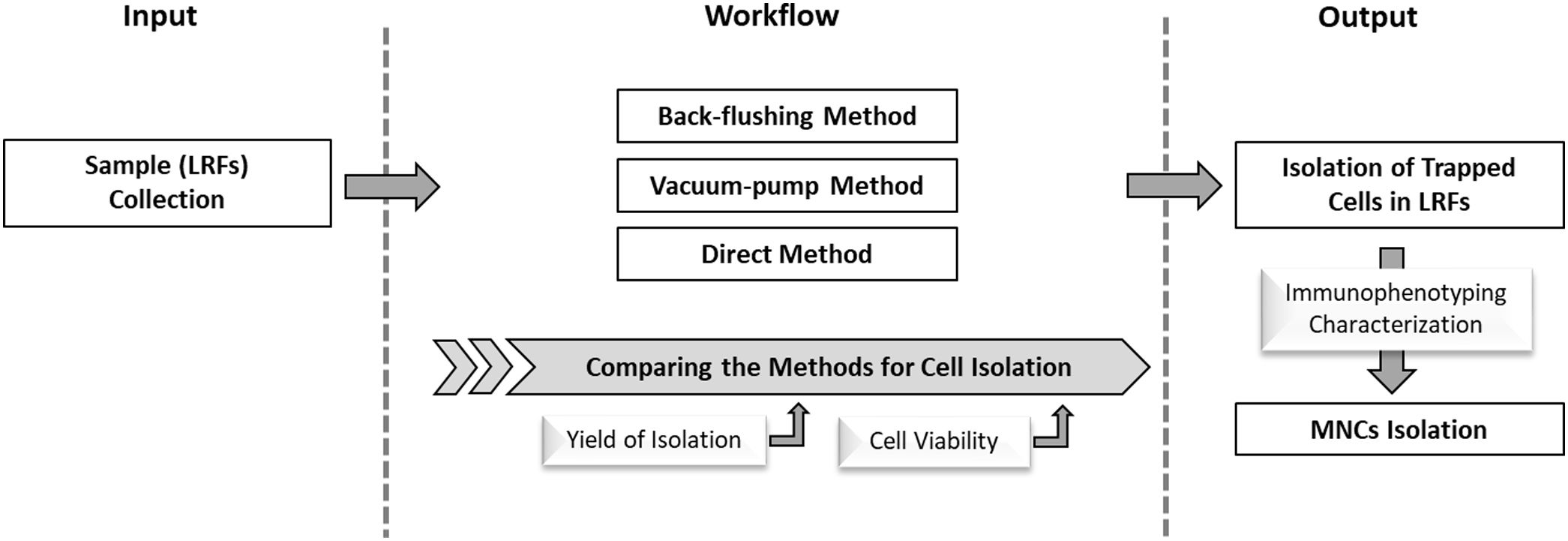
ObjectiveThe aim of this study was to evaluate various methods based on their potential to recover the peripheral blood cells from LRFs with a focus on mononuclear cells (MNCs).
MethodFor cell isolation from LRFs, three distinct methods (back-flushing, direct and vacuum pump) were compared through the calculation of the yield of isolated MNCs. The viability of extracted cells was determined by the flow cytometry technique. Moreover, the recovered MNCs were characterized regarding the presence of blood stem cell purification. The cell culture, microscopic observation, and immunophenotyping were employed to characterize the blood stem cells (hematopoietic, mesenchymal and progenitor endothelial stem cells).
ResultsThe yield of isolation obtained in the back-flushing, direct and vacuum pump methods were 17.7 ± 1.28, 17.3 ± 0.96 and 21.2 ± 0.90 percent, respectively. Although the highest potential for total blood cell recovery belonged to the vacuum pump method, the lowest cell viability (85.73 ± 4.84%) was observed in this method. However, the isolation process of the back-flushing and direct methods had less effect on cell viability. The characterization of the isolated MNCs displayed that the dominant positive phenotype was for CD34/CD45, indicating hematopoietic stem cells. In addition, the endothelial stem/progenitor cells were significantly detected as CD31/CD133 positive cells.
ConclusionAccording to our results and considering the safety and efficiency potential of each of the applied methods, the back-flushing in comparison with the other methods can be considered a suitable procedure for MNC isolation from LRFs.
The LRFs are used to specifically remove white blood cells (WBCs) from the donated whole blood samples.1 The clinical advantages of using such filters could result in the reduction of the potential harm to the leukocyte residues.2 This process can reduce the incidence and severity of febrile transfusion nonhemolytic transfusion reactions3,4, as well as the transmission of leukocyte-associated viruses, e.g., herpesviruses5 and cytomegalovirus (CMV).6,7 It has been shown that in HLA alloimmunization8 there can be secondary complications following blood transfusion containing WBCs. Moreover, from the experimental perspective, leukocytes contain enzymes and various cytokines that affect the quality and shelf life of blood products.9,10 The first generation of the filters was made of cellulose acetate and their quality of elimination depended on the filtration rate. At present, the third and fourth generation of filters (with a pore diameter of approximately 2–6 microns) are developed with the efficacy of >99% in removing leukocytes.11 Their nanofiber structure is based on a biodegradable polymer called polybutylene terephthalate. The cell isolation mechanism is a combination of physical, chemical, and biological processes. The physical construction of the nanofiber-based materials results in increasing the non-deformable cell retention. It seems that, in addition to the chemical bond, the trapped platelets also facilitate the interactions between cells and filters.9,12,13 Nonetheless, many different cells, along with their progenitors, are captured and discarded after filtration. Although the captured cells in LRFs can be considered a precious source of blood cells for many in vitro experiments, tissue engineering and nanotechnology, there are few studies of success in the extraction of the cells from the mentioned filters. The profits of leukoreduced filters include easy access to a high number of filters with a significant capacity of desired cells.11,14 The cells can be isolated in a well-organized and time-consuming process which can be performed by trained personnel. Approximately two billion cells can be isolated from the LRFs in less than eight hours in the blood donation process.15
In the present study, the isolation efficacy of the MNCs, in addition to red blood cells (RBCs), and platelets, has been tested using three extraction methods (back-flushing, direct, and vacuum pump). In the next step, the phenotype of the eluted cells from LRFs was evaluated to detect the possible presence of blood stem cells, including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and endothelial stem cells (ESCs) among MNCs. This study was approved by the ethics committee of the High Institute for Research and Education in Transfusion Medicine of the Iranian Blood Transfusion Organization with the approval ID of IR.TMI.REC.1397.016.
Materials and methodsReagents and antibodiesCell growth media of Dulbecco’s modified Eagle’s medium (DMEM; 1 g/L of d-glucose), RPMI 1640 medium and DMEM/F12 Dulbecco’s modified Eagle medium were provided by Gibco (USA) and then supplemented with 50 mg/mL of streptomycin (Sigma, USA), 50 U/mL penicillin (Sigma, USA), 2.5 mg/mL amphotericin B and 2 mM l-glutamine (Sigma, USA). Fetal calf serum (FCS) and Trypsin/EDTA (0.5%) were purchased from Gibco (USA) and collagen, from STEMCELL Technologies (Canada). Leukoflex LCR-5 was purchased from Macopharma (France). Red blood cell lysis buffer, Propidium iodide (PI), and ficoll were supplied by Sigma (USA). Monoclonal antibodies against surface markers were supplied by Dako (Denmark). The solvents were purchased from Merk (Germany).
Isolation of cells from leukoflex filtersA total number of 105 filters (35 per each group) were applied to isolate the desired cell populations. Blood sampling was carried out at the Vesal Blood Transfusion Center (Iran) by technicians and donated blood bags were stored for 2 h. To condense the blood and eliminate leukocytes, the filtration was performed using leukoflex LCR-5 filters. The LCR-5 filters were transferred to the Stem Cells Department of the Iranian Blood Transfusion and Organization for subsequent studies. To ensure that the difference in the cell recovery rate and viability were not due to the processing duration, all steps were performed on a similar schedule in a defined duration of time. To obtain a precise prospect of the MNC elution, its isolation efficiency was compared with other separatable particles (RBCs and platelets) from filters. Then, the isolated MNCs were subjected to the identification of blood stem cell content.
The maximum cell isolation potential of three different LRF methods (back-flushing, direct and vacuum pump) were compared. A buffer solution (200 cc) was used to wash the filters in all three methods. The buffer was composed of (gr) sucrose, 25; Na2EDTA, 1.8 in a phosphate buffer saline solution (PBS; Gibco, USA), in a stock of 1000 μM. The pH was adjusted to 7.2 and the solution was stored at room temperature (RT).16,17
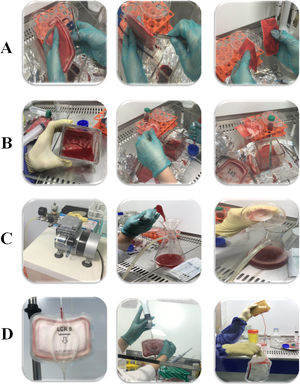
In the Direct method, the filter bases were unwrapped with sterilized scalpel inside a Class II laminar hood. The filter layers were put in a sterile dish containing buffer solution and then continuous physical pressure was used to separate cells (Fig. 1B). In the second selected method (using a vacuum pump), the filter bases were cut off in a sterile condition similar to the direct method, but the difference was that the filter layers were individually separated and put into a glass funnel placed on a flask connected to a vacuum source. Subsequently, they were washed using buffer dilution simultaneously with 150 psi/10 bar pressure generated by the vacuum pump (Fig. 1C). The back-flush is a more common method for washing and isolating cells from LRFs. Summarizing, the buffer solution is poured into a side bag and passed through filters in the reverse direction. For better isolation, air pressure was applied into the filter chamber by air syringe (60 cc) (Fig. 1D).18
Blood cell countingThe cell counting was performed in all filtration stages using the cell counter Sysmex XS 800i. Summarizing, 100 µL of cellular suspension was diluted with 900 µL of PBS and the number of cells was calculated with the following formula:19
Number of cells = Mean × Dilution factor × Volume factor
Yield of isolationThe cell isolation efficiency was calculated based on the obtained results from cell counting. Firstly, the peripheral blood sample was collected in a blood bag (450 mL) and the numbers of MNCs, RBCs and platelets were calculated in each bag prior to filtering (pre-filtration sample). After filtration, the obtained product (post-filtration sample) was re-counted for the number of cells. By comparing the cell numbers of pre- and post-samples, the number of trapped cells in a filter was determined) trapped cells). After washing the filters with the buffer solution in each method, the number of isolated cells was revealed in each method group (isolated cells). Also, the difference between the number of trapped cells and washed cells shows the number of remaining cells after washing (after wash trapped cells). According to the results, the ratio of isolated to trapped cells represents the yield of isolation from leukoflex filters and is reported as percentages.
Viability assayTo determine the effects of each isolation method on the cell viability, the flow cytometry technique was employed using propidium iodine (PI) dye. Briefly, the obtained cell products from washed filters (200 cc) were centrifuged for 10 min at 1200 g. The amount of 10 µL of PI was added to each cell suspension (5 × 105 cells in each sample) and the samples were placed in the dark for one hour at 4 °C. The erythrocytes were removed 10 min before analysis by the addition of a lysis buffer to the samples. Finally, the viability of cells in each group was estimated in comparison with the control (pre-filtration sample obtained from blood bag) using the flow cytometer Partec-PAS III and Partec software.19
Isolation of MNCsAs it has been mentioned, a suspension of isolated cells in a buffer solution (200 cc) was obtained after washing filters in three different isolation methods. The Ficoll-Paque media (density gradient: 1.077 g/mL) was used for the isolation of MNCs from the cell suspension.20
Characterization of mononuclear stem cells (MNSCs)To characterize peripheral blood stem cells (e.g., HSCs, MSCs, ESCs and their progenitors), isolated MNCs from filters were cultured in supplemented specific mediums (RPMI, DMEM/low glucose and DMEM:F12, respectively) containing 10% FCS under the conditions of 37 °C, 5% CO2 and 95% humidity. The growth medium was changed twice weekly and the cells were passaged at least three times. The surface of the tissue culture flasks was covered with collagen prior to seeding cells in order to isolate MSCs and ESCs because of their adhesiveness to the plastic surfaces. In addition, the morphological characteristics were examined by an inverted phase-contrast microscope (Leica, Germany) and immunophenotyping was performed. Briefly, cell suspension (105 cells per sample) was combined with 5 µL of monoclonal antibodies (CD31- FITC, CD34- PE, CD45- FITC, CD90-PE, CD105-FITC and CD113- PE) and incubated in the dark at 4 °C for 30 min. After washing with PBS, the cells were fixed with paraformaldehyde 1% (50 µL). Finally, the samples were immunophenotyped in comparison with the isotype control by flowcytometry (Partec-PAS III, Germany) equipped with Partec software.21,22
Data analysisA total of 35 samples were collected for cell isolation for each defined method (three groups, totalling 105 samples). The results of each group were shown as the mean ± standard deviation. The isolation efficiency and the cell viability values were compared with One-way Anova and Tukey’s Post Hoc Tests and reported in percentages. The Partec software was employed to analyze the results of immunophenotyping. All the quantitative data were statistically analyzed by the SPSS software (Version 24) and p-values lower than 0.05 were assumed as statistically significant.
ResultsEffect of applied methods on the rate of isolationThe filter structure was carefully studied to design appropriate methods for cell isolation (Fig. 1A). Eventually, three candidate methods (back-flushing, direct, and vacuum pump methods) employed for the isolation of cells trapped in the LRF (Fig. 1B–D).
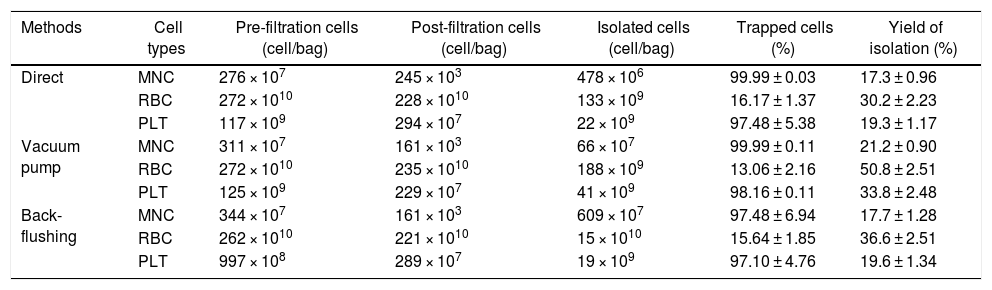
By calculating the cell number in various stages of the isolation process, the isolation efficiency of selected methods was reported (Table 1). Statistical comparison between post-filtration and isolated cells showed significant differences, confirming the recovery of target cells from LRFs (p < 0.05) (Table 1). One of the main purposes of our experiment was to find the best method for retention of MNCs from peripheral blood circulation. Regarding the calculated values in Table 1, the average yields of isolation of MNCs in the applied methods (back-flushing, direct and vacuum pump methods) were 17.7 ± 1.28, 17.3 ± 0.96 and 21.2 ± 0.90 percent, respectively. Hence, there was no difference between direct and back-flushing methods for MNC isolation and the highest yield belonged to the vacuum pump method. As mentioned, in order to have a benchmark for comparing the recovery efficiency of the methods, we calculated the number of RBCs and platelets trapped in the LRFs. It is noteworthy that the platelet isolation from filters also followed a trend similar to that of the MNSCs. According to the results, more than 97 percent of the platelets were captured in the LRFs during the filtration process (p < 0.05) (Table 1). The results show that the vacuum pump method, in terms of the yield of RBCs isolated from theLRFs, was significantly higher than the other two methods (50.8 ± 2.51%), with the back-flushing and direct methods in second (36.6 ± 2.51) and third (30.2 ± 2.23) places, respectively, (p < 0.05) (Table 1).
The cell isolation from LRFs, applying three different methods: direct, vacuum pump and back-flushing. Different parameters were considered to calculate and compare the efficiency of cell isolation among these methods. The results are obtained from 35 samples in each group and statistical significance was established at p < 0.05.
| Methods | Cell types | Pre-filtration cells (cell/bag) | Post-filtration cells (cell/bag) | Isolated cells (cell/bag) | Trapped cells (%) | Yield of isolation (%) |
|---|---|---|---|---|---|---|
| Direct | MNC | 276 × 107 | 245 × 103 | 478 × 106 | 99.99 ± 0.03 | 17.3 ± 0.96 |
| RBC | 272 × 1010 | 228 × 1010 | 133 × 109 | 16.17 ± 1.37 | 30.2 ± 2.23 | |
| PLT | 117 × 109 | 294 × 107 | 22 × 109 | 97.48 ± 5.38 | 19.3 ± 1.17 | |
| Vacuum pump | MNC | 311 × 107 | 161 × 103 | 66 × 107 | 99.99 ± 0.11 | 21.2 ± 0.90 |
| RBC | 272 × 1010 | 235 × 1010 | 188 × 109 | 13.06 ± 2.16 | 50.8 ± 2.51 | |
| PLT | 125 × 109 | 229 × 107 | 41 × 109 | 98.16 ± 0.11 | 33.8 ± 2.48 | |
| Back-flushing | MNC | 344 × 107 | 161 × 103 | 609 × 107 | 97.48 ± 6.94 | 17.7 ± 1.28 |
| RBC | 262 × 1010 | 221 × 1010 | 15 × 1010 | 15.64 ± 1.85 | 36.6 ± 2.51 | |
| PLT | 997 × 108 | 289 × 107 | 19 × 109 | 97.10 ± 4.76 | 19.6 ± 1.34 |
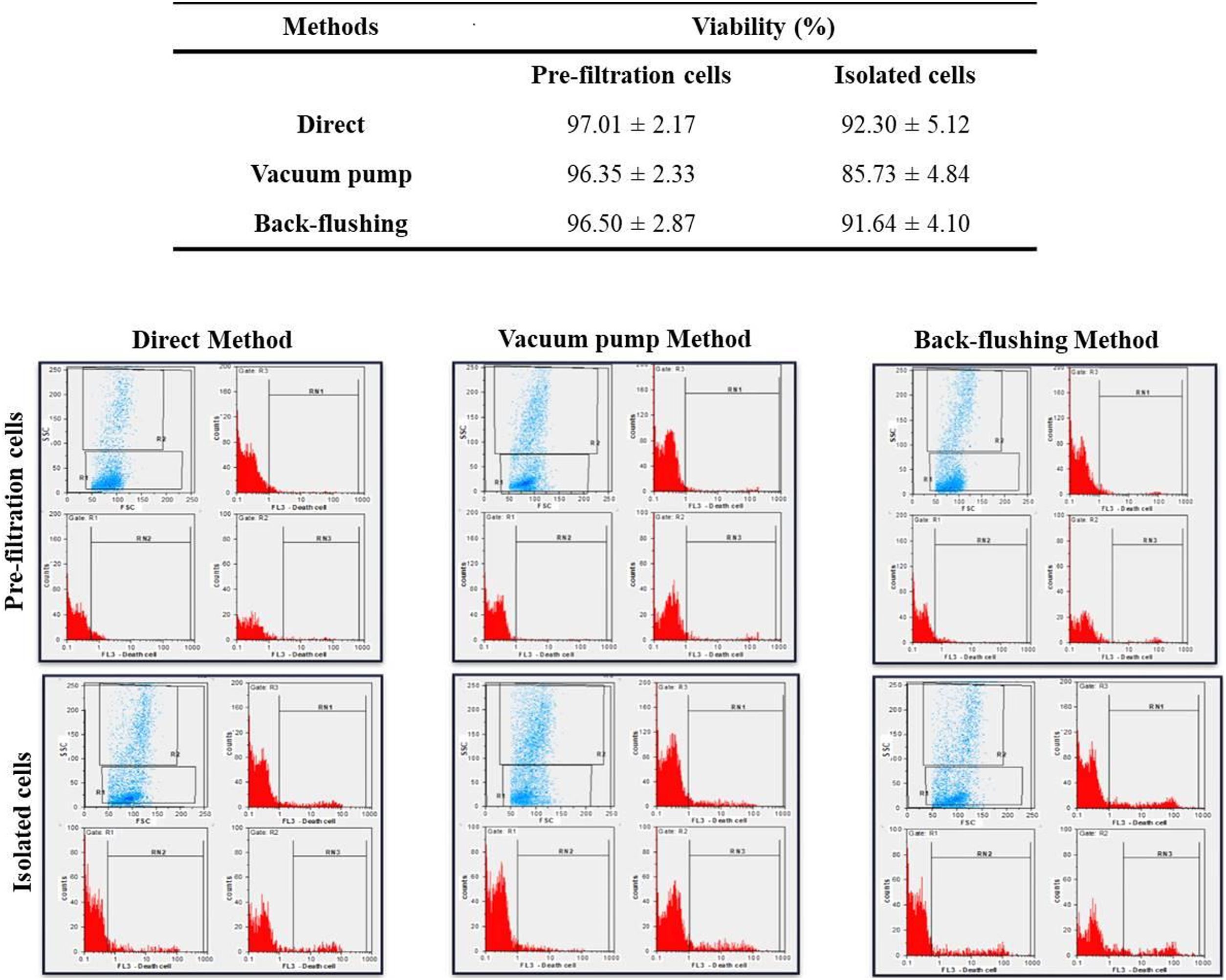
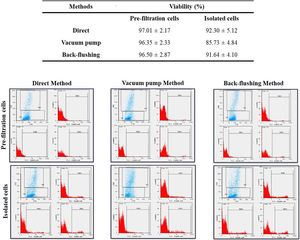
The viability of isolated MNCs, compared to the control (pre-filtration blood), was evaluated in each method. The viability of the MNCs isolated from the LRFs using the back-flushing, direct, and vacuum pump methods were 91.64 ± 4.10, 92.30 ± 5.12 and 85.73 ± 4.84 percent, respectively. The average amount of cell viability in pre-filtration samples was 96.76 ± 2.73 percent. According to the results, it can be concluded that the isolation process in the vacuum pump method had the greatest, while the direct method had the least, effect on cell viability (p < 0.05) (Fig. 2).
The flowcytometry evaluation of cell viability, following PBMNC isolation, with direct, vacuum pump and back-flushing methods, from LRFs. The experimental values were compared with pre-filtration control values. The results are expressed as mean ± standard deviation and statistical significance established at p < 0.05.
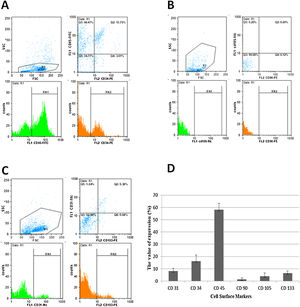
The isolation of MNCs was performed for the purpose of purifying the stem cells (HSCs, MSCs and ESCs) from peripheral blood. As described in methods & material, after ficoll separation, the characterization of MNSCs was evaluated using cell culture, morphological observation, and superficial markers of CD34 and CD45 (for HSCs), CD90 and CD105 (for MSCs) and CD31 and CD133 (for ESCs).23,24
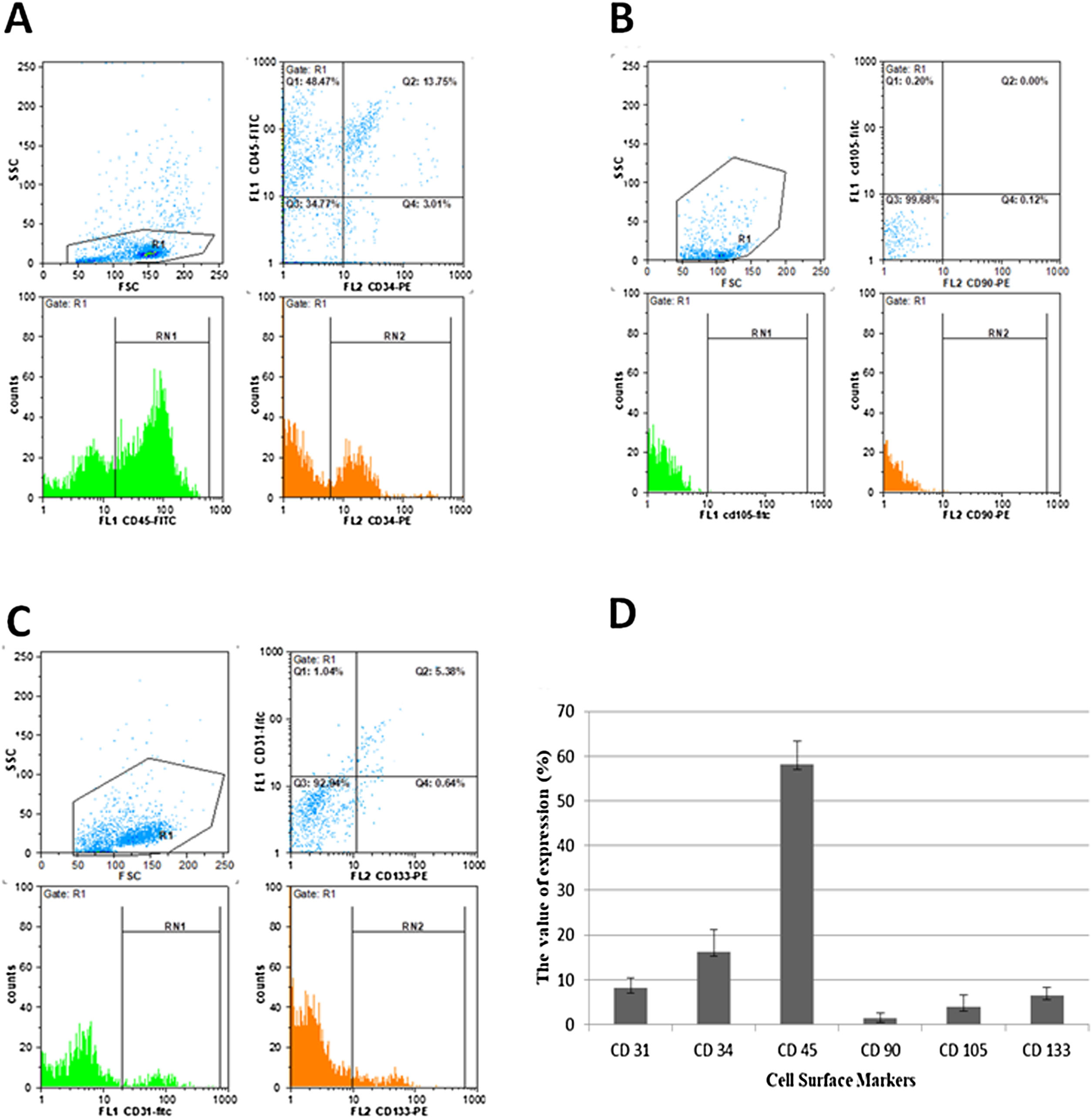
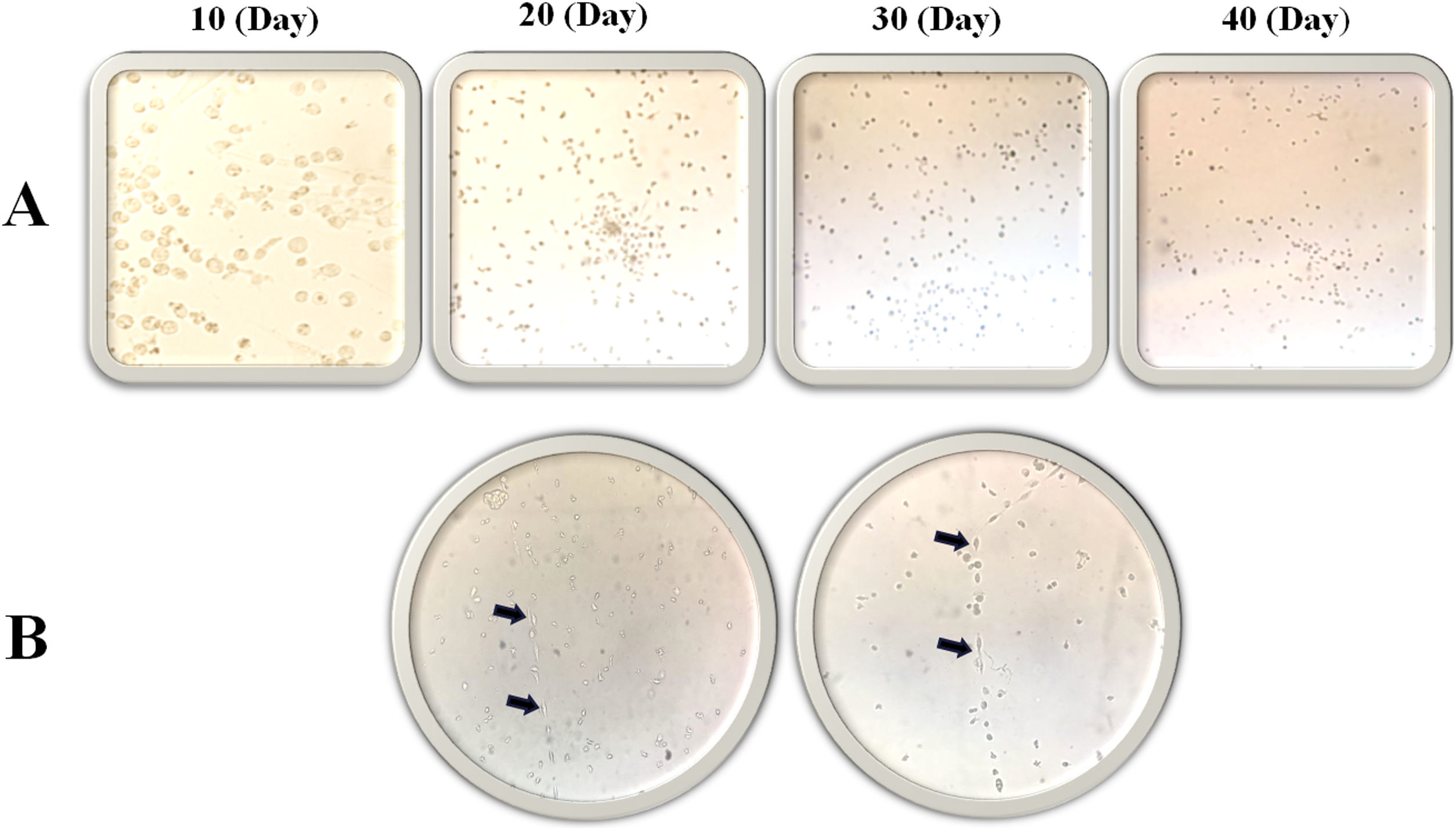
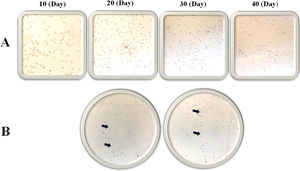
Morphology evaluation of MNCsThe results illustrated that the expression of the MSC markers was less than 5% (Fig. 3). In the first days of the culture in a specific medium for MSCs (DMEM; 1 g/L of d-glucose), the presence of fibroblastic-like colonies encouraged us to follow our study for 40 days (Fig. 4), but the lack of colony growth and the results of re-phenotyping showed that the observed colonies were not related to MSCs. The expression percentage of CD34 and CD45 in the PBMNCs were 19.23 ± 4.9 and 58.07 ± 5.3 percent, respectively. The flowcytometry analysis showed that the total count of HSCs was reported as 1.64 × 106 ± 9.75 × 104. The expression of surface markers associated with ESCs was 8.12 ± 2. 33 for CD31 and 6.5 ± 1.78 for CD133 (Fig. 3). In the culture of ESCs in DMEM/F12 Dulbecco's modified Eagle medium containing 1% heparin, structures resembling vasculogenesis (colonies of precursor cells) were observed and remained until the second week. Then the colony growth stopped and the senescence phenomenon led to cell separation from the bottom of the tissue culture flask in the third week (Fig. 4).
Immunophenotyping characteristics of cells isolated from LRFs. The isolated cells are labeled for CD31-FITC, CD34-PE, CD45-FITC, CD73-PE, CD90-FITC/PE, CD105-FITC and CD133- PE monoclonal antibodies, as described in Materials and Methods. The experiment was performed in triplicate. The CD34/45 positive cells (A), CD90/105 negative cells (B), CD31/133 positive cells (C) and surface makers expression of PBMNCs isolated from LRFs by the back-flushing method (D).
Every day, many people donate blood for hospital usage to give others a chance to survive. In blood transfusion centers, LRFs are used to remove leukocytes from whole blood to prevent the related complications in blood recipients.18,25Donated blood bags consist of a large variety of cells, among which are stem cells. After filtration, many of such cells are trapped in LRFs at blood transfusion centers and are subsequently discarded. In fact, LRFs are designed to be structurally and physically capable of trapping cells. On the other hand, peripheral blood cells also tend to attach to their nano-fibrillar networks. Hence, isolation of such cells from LRFs could be noteworthy as a new source for studies on blood components (Fig. 5).
There are very few studies about the introduction and comparison of different methods for cell isolation from LRFs and those that exist made use of methods with insufficient potential. Additionally, the cellular adhesion to filters and the mechanism of leukocyte depletion can make it very difficult to find a suitable method. In this study, we plan to determine an acceptable isolation efficiency using three designed methods. Although the isolation yield in the vacuum pump method was higher than that of the two others, it yielded the lowest viability. Its significantly reduced cell viability, compared to the other two methods, can be attributed to the higher physical pressure on samples during the procedure. It should be also noted that the safety of a method is of great importance to scientists. Therefore, the high risk of exposure to biological materials due to the unwrapping of filters and the separating of sheets is defined as a disadvantage for the vacuum pump method. Therefore, the back-flushing method takes priority over the vacuum pump and direct methods. Furthermore, there was no significant difference in the isolation yield between the direct and back-flushing methods, except for the recovery of RBCs.
Peripheral blood is known as an ideal source of stem cells for research projects.26 Accordingly, LRFs are defined as a presenter of PBMNCs which can be widely applied for therapeutic experiments, in vitro and ex vivo studies.16,27Although the increase of isolation capacity of methods gives the researcher more chance to collect peripheral blood stem cells, the direct access to the MNSCs (HSCs, MSCs and ESCs) trapped in LRFs has limitations. For example, there are contradictory reports on the isolation of MSCs from peripheral blood of different species. The MSC isolation from equine peripheral blood was possible almost in 36 percent of the samples28, while in another study of the feline model, this finding was 68 percent.29 On the other hand, in some other studies, it has been stated that peripheral blood, in comparison with bone marrow cells, cannot be used as a reliable source for MSC isolation.30 To enhance the possibility of MSC isolation, the simultaneous use of fibrin microbeads31, along with diverse buffers to decrease the cell adherence potential with other rich and specific culture mediums, can be helpful.
A number of texts have been published regarding the isolation of the hematopoietic peripheral blood stem cells according to acceptable protocols provided in this field.15,18 In the present study, the total count of recovered HSCs from filters was calculated at 1.64 × 106 ± 9.75 × 104 per filter. Considering that this amount of cells is sufficient for use in an in vitro experiment, the LRFs can be as a reliable source for the expansion of HSCs.32 In recent years, there have been many advances in the therapeutic use of isolated ESCs.33,34 Positive ESCs were detected in the suspension of isolated cells from leukoflex filters in this study. Subsequently, the emergence of the vasculogenesis formation during the culture confirms the ESC isolation. Based on our results, LRFs can be considered a novel and ideal source for the isolation of ESCs and their progenitors. It is worth mentioning that, considering the results of our study, the filters have the potential to almost completely capture platelets from the peripheral blood of donors. Since platelets are exquisite ingredients in tissue repair, regenerative medicine and wound healing, the isolation of trapped platelets and their therapeutic application is attracting much attention.35,36 However, our goal in measuring platelets and RBCs was simply to compare and evaluate the efficacy of the applied methods.
In addition to the efficiency, the safety of a technique is an important factor that was taken into account. Allogeneic hematopoietic stem cell transplantation is associated with a variety of infectious complications that can seriously threaten the health of the transplant recipient.37 Due to precise infection monitoring after blood donation, the isolated cells from LRFs are free of contaminants, which can make them more valuable. Regarding the risk of infection transmission in cell therapy, as well as in stem cell transplantation37,38, filter-extracted stem cells can be given more attention, as they constitute an appealing available source.
ConclusionSince leukoreduction filters can be considered a practical source of human peripheral blood cells (especially MNCs) for biological application, cell isolation through a non-invasive method is of utmost importance. The optimization of the isolation efficiency of a method was the aim of our study. Applied methods have provided the possibility of proper manipulation to achieve the optimal outcome. Moreover, the significant number of stem cells trapped in the LCR5 filters introduces a rich source for application in reconstructive medicine. This project is a major step forward in the transformation of waste into a source of wealth, which can be followed by other researchers.
FundingThis study was funded by the High Institute for Research and Education in Transfusion Medicine of the Iranian Blood Transfusion Organization (Tehran, Iran) and the Tarbiat Modares University (Tehran, Iran).
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the Stem Cell and Cord Blood Banking Section, as well as the Innovation Center of the Iranian Blood Transfusion Organization.
The original data were generated in the Stem Cell and Cord Blood Banking Section as well as the Innovation Center of the Iranian Blood Transfusion Organization (Tehran, Iran). Supplementary findings of this study are available from the corresponding author.