In acute myeloid leukemia (AML) therapy, venetoclax (ABT-199), a selective inhibitor of BCL2,1 has been introduced in clinical practice, presenting promising results in unfavorable or elderly AML patient groups.2–4 BCL2 family proteins are key apoptosis regulators, play an important role in the AML pathogenesis and its components are classified into BCL2 homology (BH) multidomain anti-apoptotic, BH multidomain pro-apoptotic, activation BH3-only pro-apoptotic or sensitizer BH3-only pro-apoptotic.5 Recently, several mechanisms of resistance to venetoclax have been identified, including increased expression of MCL1, BCL2L1 (also known as BCL-XL), and BCL2A1.6–8 Obatoclax (GX15-070) is a BH3 mimetic and acts as a pan-inhibitor of anti-apoptotic members of the BCL2 family.9 Mechanically, venetoclax selectively binds to the BH3-binding groove of BCL2 protein and inhibits its function,1 while obatoclax binds to a hydrophobic pocket within the BH3-binding groove of multiple anti-apoptotic members of the BCL2 family (i.e. BCL2, BCL2L1, and MCL1), inhibiting their functions.10 In the present study, we characterized the sensitivity to venetoclax and obatoclax, as well as, the expression of the main BCL2 family genes in AML cellular models.
MOLM13, MV-4-11, Kasumi 1, and OCI-AML3 leukemia cell lines were used. For cell viability assay, a total of 2×104 viable cells were plated per well in the presence of the vehicle, venetoclax (ranged 0.1–50μM), or obatoclax (0.003–3μM). After incubation for 24, 48, or 72h at 37°C, 5μg/mL of methylthiatetrazolium (MTT) (Sigma–Aldrich, St Louis, MO, USA) reagent was added, and the cells were incubated at 37°C for 4h. The reaction has stopped by the addition of 100μL of 0.1N HCl in isopropanol and the absorbance at 570nm was measured using an automatic plate reader.11 The IC50 values were calculated by non-linear regression using GraphPad Instat 5 (GraphPad Software, Inc., CA, USA). For autonomous colony formation assay, a total of 1×103cells/mL were seeded in growth factor-free methylcellulose medium (MethoCult 4230; StemCell Technologies Inc., Vancouver, BC, Canada) in the presence of the vehicle, venetoclax, or obatoclax for 10 days (long-term exposure). Colonies were detected by adding 150μL (5mg/mL) of MTT reagent and scored by Image J quantification software (U.S. National Institutes of Health, Bethesda, MD, USA). For gene expression analysis, total RNA was obtained using TRIzol reagent and cDNA was using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, San Jose, CA, USA). Quantitative PCR (qPCR) was performed using a QuantStudio 3 and SybrGreen System (Thermo Fisher Scientific) using specific primers for 19 BCL2-related genes (Supplementary Table 1). HPRT1 and ACTB were used as reference genes. Relative quantification values were calculated using the 2−ΔΔCT equation and the median of ΔCT from all cell lines was used as calibrator for each gene. Statistical analyses were performed using GraphPad Instat 5 (GraphPad Software, Inc., San. Diego, CA, USA). For comparisons, ANOVA and Bonferroni post-test were used. p-value <0.05 was considered statistically significant.
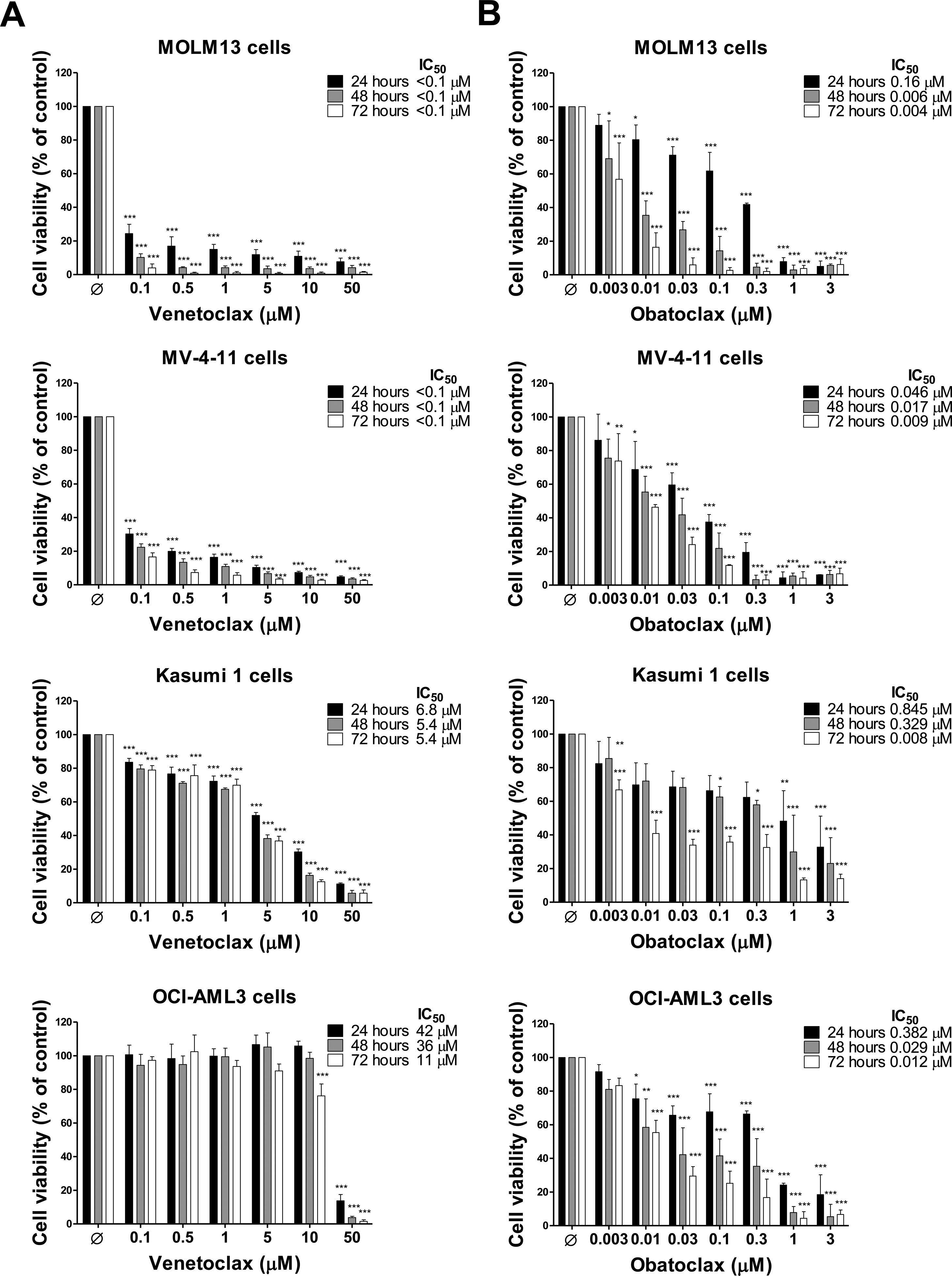
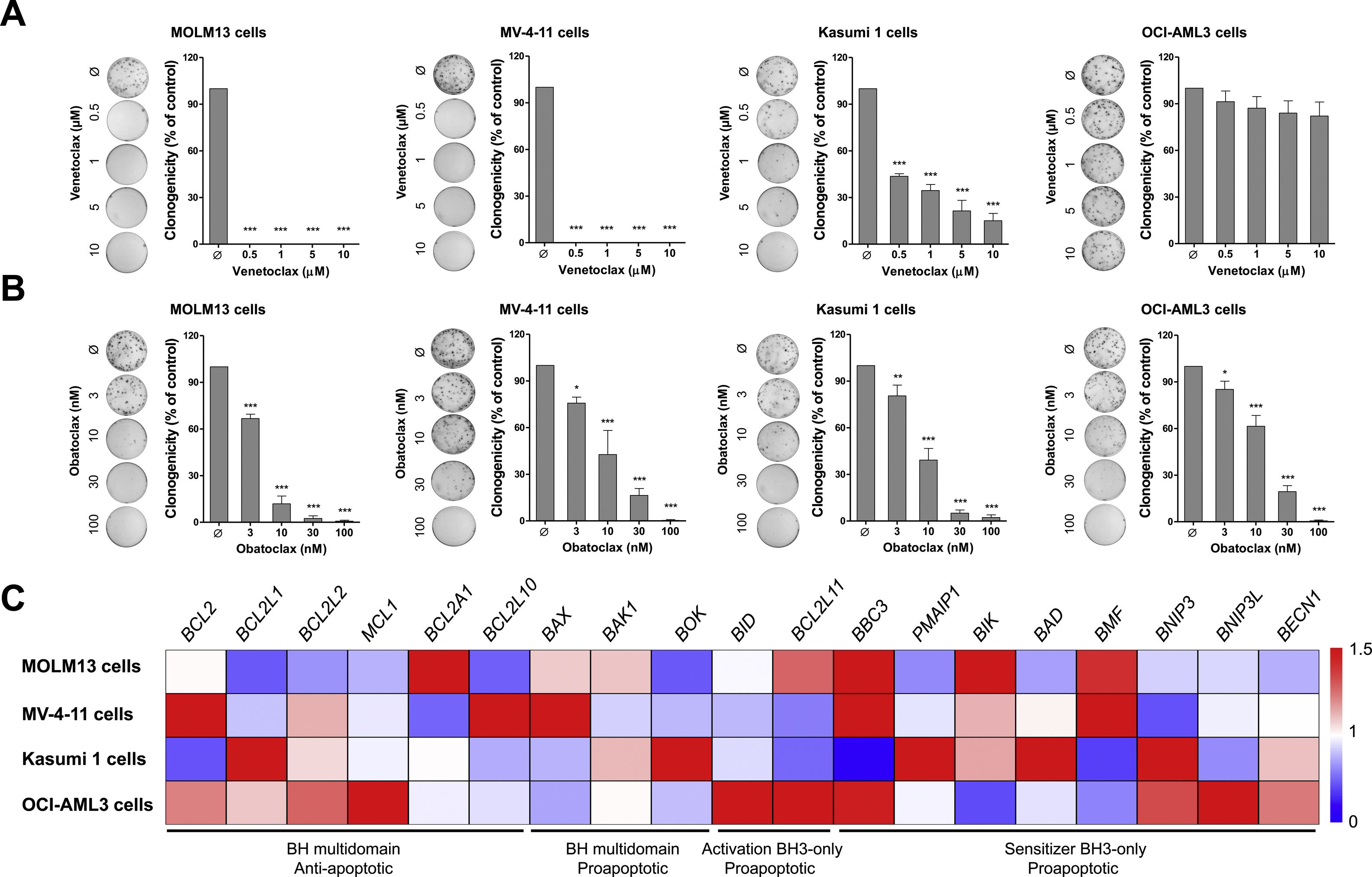
Cell viability assays identified two venetoclax-sensitive AML cell lines (MOLM13 and MV-4-11) with IC50 <0.1μM, one AML cell line with intermediate venetoclax-resistance (Kasumi 1) with IC50 range 5.4–6.8μM and one AML cell line venetoclax-resistant (OCI-AML3) with IC50 range 11–42μM (Figure 1A). These results are in agreement with the previous studies12–14 and provide novel insights on time-dependent effects. On the other hand, all AML cell lines showed similar sensitivity to the obatoclax, mainly within 72h. The IC50 ranges for the MOLM13, MV-4-11, Kasumi 1, and OCI-AML3 cells were 0.004–0.16μM, 0.009–0.046μM, 0.008–845μM, and 0.012–0.382μM, respectively (Figure 1B). Colony formation assays corroborate cell viability findings, indicating that obatoclax reduces cell viability of AML cell lines independently of their sensitivity to venetoclax (all p<0.05; Figure 2A).
Dose- and time-effects of venetoclax and obatoclax in acute myeloid leukemia cell lines. Dose- and time-response cytotoxicity were analyzed by methylthiazoletetrazolium (MTT) assay for MOLM13, MV-4-11, Kasumi 1, and OCI-AML3 cells treated with vehicle (Ø) or increasing concentrations of venetoclax (0.1, 0.5, 1, 5, 10, and 50μM) (A) or obatoclax (0.003, 0.01, 0.03, 0.1, 0.3, 1, and 3μM) (B) for 24, 48, and 72h. Values are expressed as the percentage of viable cells for each condition relative to vehicle-treated controls. Results are shown as the mean±SD of at least four independent experiments. The p values and cell lines are indicated in the graphs; *p<0.05; **p<0.01; ***p<0.001; ANOVA and Bonferroni post-test.
Obatoclax effectively reduces clonal growth independently of BCL2-related molecular background and venetoclax sensitivity in acute myeloid leukemia cell lines. (A) Colonies containing viable cells were detected by methylthiazoletetrazolium (MTT) after 10 days of culture of MOLM13, MV-4-11, Kasumi 1, and OCI-AML3 cells treated with vehicle or increasing concentrations of venetoclax (0.5, 1, 5, and 10μM) or obatoclax (3, 10, 30, and 100nM) and normalized to the corresponding vehicle-treated controls (Ø). Colony images are shown for one experiment and the bar graphs show the mean±SD of at least three independent experiments. *p<0.05; **p<0.01; ***p<0.001; ANOVA and Bonferroni post-test. (B) Gene expression heatmap from qPCR analysis for 19 BCL2-related genes in MOLM3, MV-4-11, Kasumi 1, and OCI-AML3 cells. The mRNA levels are normalized to the median of ΔCT from all AML cell lines for each gene. BCL2 homology (BH) multidomain anti-apoptotic, BH multidomain pro-apoptotic, activation BH3-only pro-apoptotic or sensitizer BH3-only pro-apoptotic genes are indicated and genes are reported according to Human Genome Organization (HUGO) Gene Nomenclature Committee (HGNC). Three independent samples for each cell line were used for the analysis; blue indicates repressed mRNA levels and red elevated mRNA levels.
In the molecular scenario, Kasumi 1 presented higher levels of BCL2L1, and OCI-AML3 cells presented higher expression of anti-apoptotic BCL2 members, especially MCL1. In fact, the inhibition of MCL1 increased the sensitivity to venetoclax in OCI-AML3 cells, similarly to overexpression of BCL2L1 reduced the sensitivity to venetoclax in HL60 cells,14 which supports our findings in Kasumi 1 cells. Other findings that draw attention are the increased BMF levels and reduced BNIP3 levels in the sensitivity compared with venetoclax-resistant AML cell lines (Figure 2B and Supplementary Table 2). Of note, obatoclax was found to bind to venetoclax resistant-related BCL2 members, including MCL1, BCL2L1, and BCL2L2,9,15 which may explain its high efficiency and potency regardless of the BCL2-related molecular profile observed, as well as, the previous venetoclax sensitivity in AML cell lines. A limitation of the present study is that the expressions of the BCL2-related genes were investigated based on the RNA measurements, thus, the functional protein levels of these targets may be subject to post-transcriptional (e.g. microRNAs, long non-coding RNA) or post-translational (e.g. protein degradation) changes, which should be taken into account in future studies.16,17
In phase II clinical trial, obatoclax as a single agent was not effective in an unselected group of treatment-naïve elderly AML patients. The authors further suggested that new studies may reveal obatoclax activity in select subgroups of AML patients.18 In this sense, our preliminary data suggest that the high expression of anti-apoptotic members of the BCL2 family may be used as molecular markers for predicting response to BH3 mimetics, thus, future clinical trials could consider this assessment.
In summary, our results indicated that obatoclax reduces cell viability in AML cells, independently of their sensitivity to venetoclax, suggesting that pan-BCL2 inhibition overcomes intrinsic resistance in AML cellular models. These findings provide pharmacological insights for additional investigation of molecular mechanisms involved in intrinsic resistance to venetoclax and highlighted obatoclax as a potential therapeutic option in this context.
AuthorshipK.L. participates of acquisition of data, analysis and interpretation of data, and drafting the article. H.P.V. participates of acquisition of data, analysis of data, and editing the article. J.A.E.G.C. and J.C.L.S participate of analysis of data and editing the article. L.L.F.P. and E.M.R. participate of conception of the study, interpretation of data, and critically revising the article. J.A.M-N. participates of the conception and design of the study, acquisition, analysis and interpretation of data, and drafting the article. All authors final approval of the version of the manuscript.
FundingThis study was supported by the São Paulo Research Foundation (FAPESP) [#2019/23864-7, #2018/19372-9, #2019/01700-2] and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [#402587/2016-2].
Conflicts of interestThe authors declare no conflicts of interest.