Bortezomib is a proteasome inhibitor, which has radically changed the treatment and prognosis of patients with multiple myeloma.
Its mechanism of action is based on a reversible inhibition of proteasome subunit 26s, thereby inhibiting its chymotrypsin activity. This leads to the accumulation of an inhibitor of nuclear factor kappa B (NFκB). Inhibition of this factor diminishes the expression of adhesion molecules, as well as growth, survival and angiogenic factors, increasing the number of misfolded proteins promoting apoptosis of malignant cells.1
Its most common side effects are hematological toxicity, mainly thrombocytopenia, gastrointestinal toxicity (nausea, vomiting, diarrhea, constipation) and peripheral neuropathy.
Pancreatitis due to bortezomib is very rare, with an estimated incidence of 0.1–2%,2 with only isolated reports in the literature.
Bortezomib has proven to be especially beneficial in patients with multiple myeloma with renal involvement and the 17p deletion.
The presence of renal failure implies poor prognosis and decreased survival in multiple myeloma. Bortezomib backbone treatments are the choice therapy in patients with renal injury, showing that 20–30% of patients on dialysis recovered renal function between the 2nd and 3rd cycle.
We report two patients with newly diagnosed multiple myeloma who developed pancreatitis as an adverse event during treatment with bortezomib.
Case 1A 58-year-old male with a history of high blood pressure, acute myocardial infarction and Chagas disease was referred to the hospital because of renal failure. The patient was diagnosed with multiple myeloma, lambda light chain disease, international staging system (ISS) l and fluorescent in situ hybridization (FISH) positive for the deletion of 17p. He had renal involvement with proteinuria in nephrotic range and a pathological fracture of D7.
He started treatment with CyborD, receiving subcutaneous Bortezomib (1.3mg/m2) on Days 1, 4, 8 and 11; Cyclophosphamide (300mg/m2) on Days 1, 8 and 15 and 40mg dexamethasone the day before and after bortezomib.
Four days after completing the 2nd cycle, the patient was admitted to the emergency room for confusion, asthenia and progressive fatigue. On physical examination, the patient was in bad general condition (Glasgow 3/15) with low blood pressure and respiratory failure.
The complete blood count and chemistry panel showed a hemoglobin level of 10g/dL, platelets 135.9×109/L, leukocytes 20.528×103/μL, creatinine 4.2mg/dL, uremia 202mg/dL, phosphorus 18mg/dL, amylase 1032mg/dL, lipase 1990mg/dL and glucose 900mg/dL. A computed tomography (CT) scan was performed with evidence of an infectious inflammatory process in both lungs.
The clinical presentation was consistent with septic shock secondary to respiratory infection and acute pancreatitis associated with diabetic ketoacidosis. The patient started antibiotic treatment, mechanical ventilation, inotropic support and hemodialysis.
The patient had no history of alcoholism or hypertriglyceridemia and the abdominal ultrasound, viral serology and serum calcium levels were normal, thus excluding the main causes of pancreatitis.
In the absence of another obvious cause, the elevation of pancreatic enzymes was interpreted as a possible adverse effect associated with the use of bortezomib, which was suspended, or secondary to sepsis.
The patient evolved with improvement of the septic shock, with progressive decrease of pancreatic enzymes.
Thirty days after admission it was decided to restart chemotherapy treatment with low doses of bortezomib (1mg/m2) and daily monitoring of amylase and lipase levels.
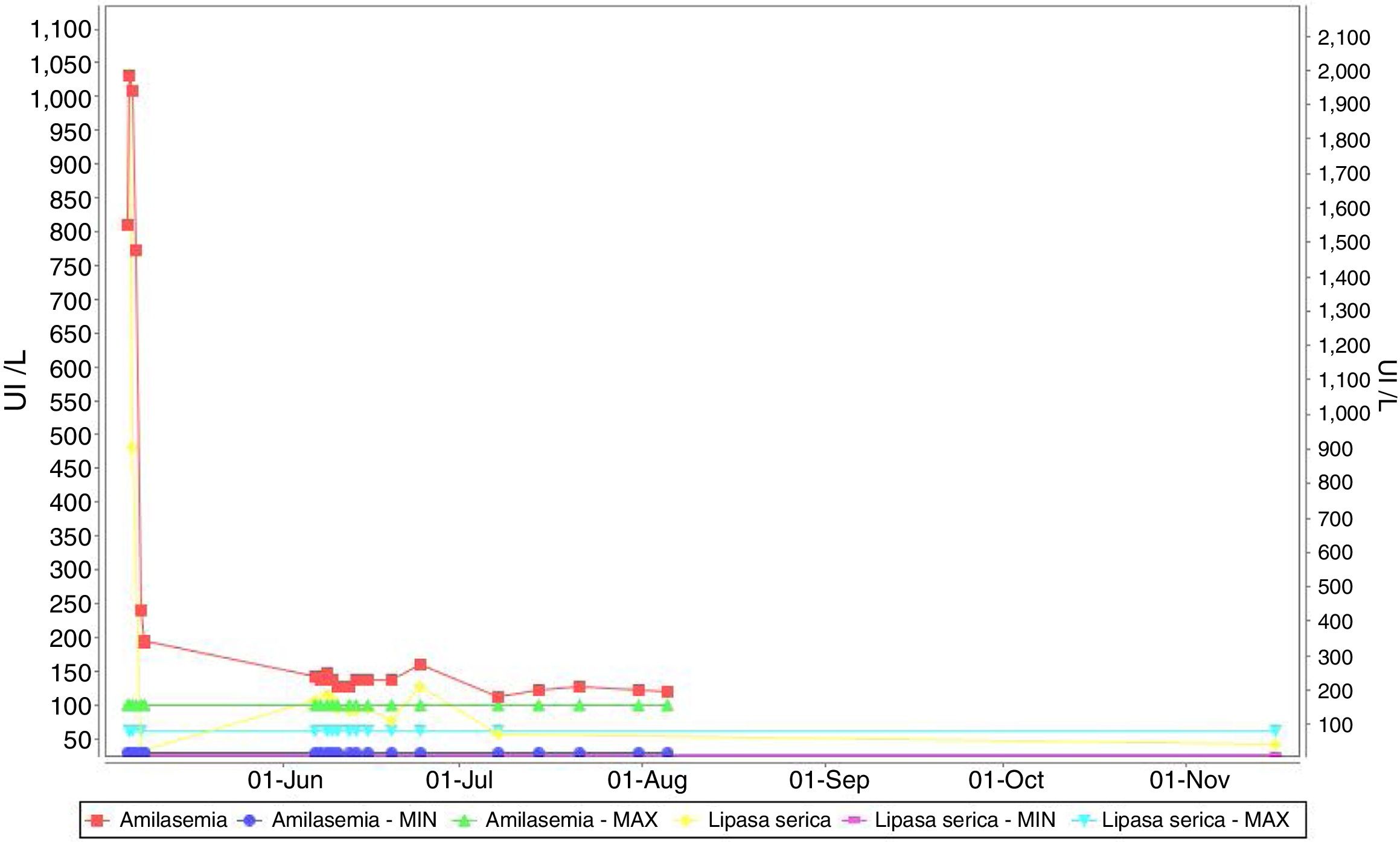
An increase of amylase and lipase twice above normal level (Figure 1) was noted coincidently with the administration of bortezomib but without associated clinical symptoms. The patient completed four cycles of treatment achieving a partial response and could discontinue dialysis with frank improvement of renal function. An autologous bone marrow transplant was performed.
Currently the patient is five months after transplantation, with relapse of the disease and planning to start second line therapy.
Case 2A 63-year-old female with a history of rheumatic fever in childhood, colonic diverticulosis and cholecystectomy was admitted to our hospital complaining of pain in the left hemithorax associated with anemia and kidney failure.
She was diagnosed with multiple myeloma, immunoglobulin G kappa, ISS 3 with bone and renal involvement, anemia and 17p deletion.
She began treatment with subcutaneous bortezomib (1.3mg/m2) on Days 1, 4, 8 and 11, dexamethasone (40mg) on the days before and after the bortezomib and thalidomide (100mg per day).
Three days after receiving the first dose of bortezomib she complained of abdominal pain. Laboratory tests showed amylase 565IU/L, lipase 1060IU/L, aspartate transaminase (AST) 88IU/L, and alanine transaminase (ALT) 201IU/L.
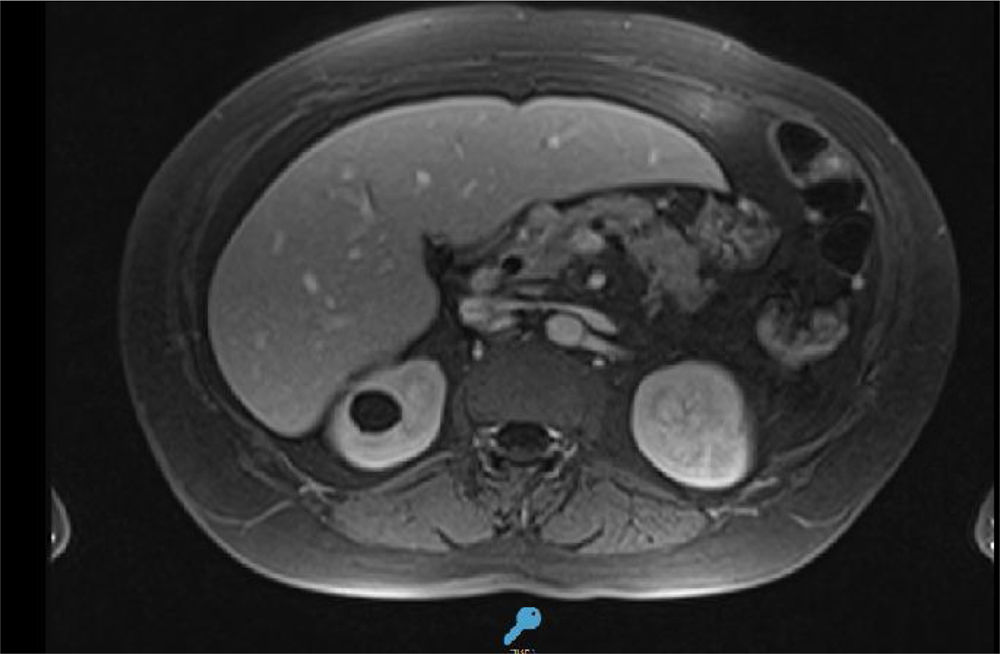
A magnetic resonance evidenced enlargement of the cephalic portion of the pancreas and parenchymal enhancement. It was associated with an alteration of the surrounding fat with small amount of free fluid (Figure 2).
The patient's symptoms and clinical findings were consistent with acute pancreatitis. The most common causes were ruled out, so it was suspected to be an adverse effect associated with bortezomib. Analgesia and bowel rest were indicated, with improvement of the patient's clinical condition and normalization of pancreatic enzymes.
Within ten days after the first dose of bortezomib, it was decided to restart chemotherapy with reduced doses of bortezomib (1mg/m2) and strict monitoring of pancreatic enzymes. A slight increase in pancreatic enzymes was evidenced after bortezomib administration, but the patient remained asymptomatic so it was decided to continue treatment with the reduced dose.
The patient completed four cycles of bortezomib with partial response and improvement of renal involvement, and underwent an autologous bone marrow transplantation.
DiscussionThe diagnosis of acute pancreatitis requires two of the following criteria: (1) characteristic abdominal pain, (2) amylase and lipase increased three times above normal value and (3) characteristic findings of acute pancreatitis on CT.
Although pancreatitis secondary to drugs is still considered a rare side effect, most studies conclude that it is the third most common cause after gallstones and alcohol consumption.3
Bortezomib pancreatitis is a rare but potentially serious complication,4 so it is important to consider it in patients receiving this treatment. The diagnosis is based on exclusion of the most common causes of pancreatitis.5,6
In all cases reported in the literature, alterations of pancreatic enzymes occurred a few days after the administration of bortezomib and in some patients, as in our first case, imaging studies were normal, which does not exclude the diagnosis.7
The mechanism of toxicity is not fully understood but may be related to direct drug toxicity and/or allergic and immunological mechanisms.8,9
It is known that kidney failure is a frequent and severe complication in patients with multiple myeloma and recovery of renal function is associated to better survival. Bortezomib achieves quick responses, and decreases the time of exposure of nephrons to light chains. It does not require dose adjustment in renal dysfunction and it is the treatment of choice in these patients.10
In the two cases reported, patients had impaired renal function and deletion of 17p, two conditions in which bortezomib is especially useful, therefore it was decided to re-challenge the patients with lower doses of bortezomib with strict monitoring of chemistry parameters.
Although a slight increase in pancreatic enzymes was observed at the 1mg/m2 bortezomib dose, patients did not develop symptoms and were able to continue and finish treatment.
Only half of the other cases reported in the literature restarted bortezomib, but it was at the same initial dose and all of them had to discontinue treatment because or recurrence of symptoms.
We present these cases to illustrate how we managed this complication and to show that it is possible to continue bortezomib treatment adjusting the dose and making frequent monitoring of pancreatic enzymes.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Dr. Victoria Otero for her contributions to this article.