Ferric carboxymaltose (FCM) is a new parenteral iron product and the first of the new agents approved for rapid and high-dose replenishment of depleted iron stores.1,2 FCM is an iron complex that consists of a ferric hydroxide core stabilized by a carbohydrate shell and its properties permit the administration of large doses (15mg/kg; maximum of 1000mg/infusion) in a single and rapid infusion without the requirement of a test dose.1,3–5 Moreover, FCM is a stable complex with the advantage of being non-dextran-containing and with a very low immunogenic potential and therefore the risk of anaphylatic reactions is low.1,3,4
FCM is, therefore, an effective option in the treatment of iron-deficiency anaemia (IDA) in patients for whom oral iron preparations are ineffective or cannot be administered. The goals of treatment in IDA include restoring haemoglobin (Hb) to normal levels, replenishing iron stores and normalizing red cell indices. Additional goals are to relieve anaemia-related symptoms and improve health-related quality-of-life (HR-QOL).1–4
FCM treatment results in transient elevations in serum iron, serum ferritin and transferrin saturation, and, ultimately, in the correction of Hb levels and replenishment of depleted iron store in several 6–12-week, randomized, open-label, controlled, multicentre trials in various patient populations, including those with inflammatory bowel disease, heavy uterine bleeding, postpartum IDA or perioperative anaemia, and those with chronic kidney disease.3–8 FCM also improved self-reported patient global assessment, NYHA functional class and exercise capacity in patients with heart failure and iron deficiency in the FAIR-HF and CONFIRM-HF trials.9,10
In most trials, patients received either FCM equivalent to an iron dose of ≤1000mg (or 15mg/kg in those weighing <66kg) administered over ≤15min (subsequent doses administered at 1-week intervals) or oral ferrous sulfate at a dose equivalent to 65mg iron three times daily or 100mg iron twice daily. FCM was considered to be as least as effective as ferrous sulfate with regard to changes from baseline in Hb levels or the proportion of patients achieving a haematopoietic response at various timepoints. In general, improvements in Hb levels and, particularly, the replenishment of depleted iron store were more rapid with FCM than with ferrous sulfate. Recipients of FCM demonstrated improvements from baseline in serum ferritin levels and transferrin saturation, as well as improvements from baseline in HR-QOL assessment scores.1,3–8
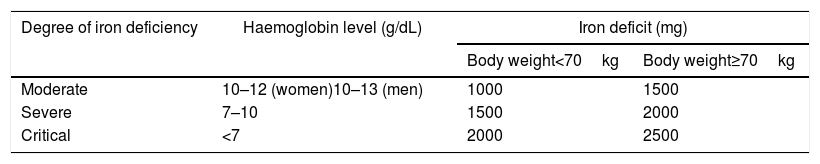
Ganzoni's formula captures the total body iron deficit in milligrams (body weight in kg×[target Hb−actual Hb in g/dL]×0.24+500).11 However, the formula is inconvenient, prone to error, inconsistently used in clinical practice, and underestimates iron requirements.3,12 The FERGIcor trial6 compared a novel and simple scheme (Table 1) with the Ganzoni-calculated dosing in anaemic patients with IBD. The simple FCM dosing regimen showed better efficacy and compliance, as well as a good safety profile, compared with the Ganzoni-calculated iron sucrose dose regimen. In this clinical trial setting, the simple scheme has only been used for dosing of FCM, however, in clinical practice, it is also used for dosing of other intravenous iron compounds. Limitations of this scheme include patients with Hb below 7.0g/dL, who likely need an additional 500mg. Also, the estimation of iron needs in iron deficiency without anaemia is not covered. A minimum of 500–1000mg should be considered.14,15
Estimated total iron deficit (mg elemental iron) based on hemoglobina and body weight.13
| Degree of iron deficiency | Haemoglobin level (g/dL) | Iron deficit (mg) | |
|---|---|---|---|
| Body weight<70kg | Body weight≥70kg | ||
| Moderate | 10–12 (women)10–13 (men) | 1000 | 1500 |
| Severe | 7–10 | 1500 | 2000 |
| Critical | <7 | 2000 | 2500 |
Simplified scheme for estimation of total iron requirements.13
The maximum recommended cumulative dose of Ferinject is 1000mg of iron (20mL Ferinject) per week.
This novel dosing scheme, based on many clinical trials and observational data on high dose IV iron administration with FCM and low molecular weight iron dextran, was approved and has been increasingly utilized in the US, EU, Brazil as well as in many other countries, can equally be utilized as a simple dosing guide for other patient groups and iron formulations that can be given at doses of 1000mg per administration for efficient and rapid iron replenishment.1,2
In response to IV iron administration, serum ferritin is greatly elevated for the first 8 wk after infusion. Therefore, ferritin should be monitored only after 8–12 wk, and in case of iron overload (TSAT>50%), treatment should be adjusted accordingly.1,2,6,7
Conflicts of interestThe authors declare no conflicts of interest.