Patients with benign or malignant blood disorders, who require allogeneic stem cell transplantation and lack an identical human leukocyte antigen HLA identicalHL sibling donor, could be transplanted with hematopoietic stem cells from unrelated adult or umbilical cord donors. However, in our country, both approaches are costly and time-consuming options.
MethodsOver the last few years, haploidentical modalities have been investigated as an alternative donor source, showing similar results to those obtained with identical HLA donors. We started using T-cell-replete haploidentical with post-transplant cyclophosphamide in 2012 and we presented our experience with patients undergoing haploidentical ransplantation compared to SIB.
ResultsSince January 2012 to date, 91 allogeneic transplants have been performed, of which 49 were haploidentical and 42 were HLA identical. The mean age of the patients was 35 years (range: 17–62). The mean CD34/kg×106 infused per group was 5.93 and 5.89, respectively. Time to granulocyte and platelet engraftment was 11 and 15 days, respectively, for haploidentical, and 12 and 14 days, respectively, for HLA identical (p=0.10). The 100-day cumulative incidence of global acute GVHD was 34% for haploidentical and 29% for SIHLA identical (p=0.9). The 2-year overall global graft-versus-host disease was 43% for haploidentical and 41% for HLA identical (p=0.8). Overall survival, relapse, and transplant and relapse-related mortality were similar between both groups.
ConclusionOur experience showed that haploidentical has similar outcomes to those obtained with HLA idential and can be performed in our country safely.
Allogeneic hematopoietic transplantation (ALOTH) is one of the curative strategies for various diseases such as acute leukemias, high-grade lymphomas, severe hemoglobinopathies and acquired or hereditary medullary aplasia.1–3 ALOTH promotes the replacement of the defective hematopoiesis and graft versus leukemia effect. The probability in finding an identical related HLA donor (SIB) is nearly 25%, thus making the detection of alternative donors a priority.2 Different transplantation modalities have been developed to perform ALOTH from alternative donors, such as identical unrelated HLA donors or identical HLA umbilical cord blood. These modalities have limited use in adults in our country because they require prolonged and expensive time to search for compatible donors at international centers.4 Umbilical cord transplantation also presents the slow recovery from hematopoiesis and high rates of infections.5 During the last decade, the haploidentical transplant modality (HAPLO) was developed, allowing for the treatment of patients with family donors who partially share HLA haplotypes, currently making it possible to perform grafts from partially compatible siblings, sons, or parents. To perform ALOTH HAPLO, there are alternatives, such as pharmacological lymphodepletion or the use of replete T-cell transplants with post-transplant cyclophosphamide.4 From 2000 to 2012, our transplant program used unrelated ALOTH and umbilical cord as alternatives for patients without SIB. However, since the development of HAPLO, we adopted this modality as a preferential alternative donor by using the non-depleted T-cell grafts with post-transplant cyclophosphamide. In this study, we showed our experience with adult patients treated with ALOTH HAPLO and SIB since 2012.
Materials and patientsPatientsWe performed a retrospective analysis of all patients undergoing ALOTH in our program from 2012 to 2018. The transplant database and electronic medical records were reviewed to obtain epidemiological, clinical and laboratory characteristics, survival times, and complications. The main objective was to compare the results obtained with HAPLO and SIB modalities in terms of engraftment, transplant-related mortality, morbidity, the response obtained, relapse, the incidence of graft-vs-host disease (GVHD), and global and event-free survival. The ethical committee of our institution approved this retrospective analysis.
Pre-transplant evaluationThe patients who were candidates for ALOTH were evaluated with spirometry and pulmonary diffusion (DLCO), echocardiography and adrenal, thyroid, parathyroid, and gestational analyses. All patients were tested for herpes virus, hepatotropic virus, retrovirus, measles, rubella, mumps, toxoplasma, and parasitic stool. The disease status prior to ALOTH was determined with bone marrow sampling to classify leukemia cases into complete remission when there was an absence of immature cells by cytology and flow cytometry; partial remission, when there was the presence of immature cells, but to a lesser extent than at diagnosis; and refractory disease or relapse, when there were more than 5% blast immature cells. In lymphoma patients, complete remission was established by PET-CT, without evidence of active disease within a period not exceeding one month prior to transplantation. In patients with aplastic anemia or non-transformed myelodysplasia, ALOTH was performed with active disease. The donor selection was performed with HLA A, B, C, and DR genotyping in intermediate or high resolution, depending on the type of transplant; in the cases of haploidentical transplantation, donor selection was complemented with specific donor anti-HLA antibodies. In patients who have anti-HLA antibodies, our program performs desensitization, as reported, with intravenous immunoglobulin, rituximab, plasmapheresis, and autologous stem cell collection for the hematopoietic recovery, in the case of engraftment failure.6
ConditioningThe patients were treated with the myeloablative conditioning (MA) and reduced intensity (AIR) scheme, according to our previously reported data.7 In summary, our program has no chronological age limit for ALOTH with conditioning chemotherapy, adjusted according to the functional status of the patient. The myeloablative scheme consisted of intravenous (IV) cyclophosphamide 60mg/kg each day for 2 days and 1320cGy of total body irradiation fractionated in 3 or 4 days. Reduced intensity regimen for lymphoma patients consisted of cyclophosphamide 50mg/kg as a single dose, fludarabine IV 40mg/m2 every day for 5 days, and total body irradiation 200cg as a single dose. For leukemia or myelodysplasia, patients were given cyclophosphamide EV 50mg/kg as a single dose, fludarabine EV 40mg/m2 every day for 5 days, and busulfan EV 3.2mg/kg for 2 days. In patients with medullary aplasia, the same MA or AIR schemes were used along with thymoglobulin.
Prophylaxis of GVHDIn patients who received SIB, the GVHD prophylaxis was methotrexate EV 15mg/m2 day +1; 10mg/m2 on days 3, 6, 9, and 12, and cyclosporin 3mg/kg every 12h IV or oral route (OR) for serum levels between 200 and 400ng/μL. In patients with HAPLO, the prophylaxis of GVHD was tacrolimus 0.03mg/kg EV or VO, mycophenolate 1g every 8h VO, and cyclophosphamide post-transplant 50mg/kg on days +3 and +4, with an equivalent dose of mesna. Immunosuppressive treatment with tacrolimus or cyclosporine was maintained for the necessary time, according to the evolution of GVHD.
Mobilization of hematopoietic precursorsOnce the patient was on the day before ALOTH, the hematopoietic precursor collection from the donor was performed, after stimulation with 600μg of filgrastim subcutaneous administration (sc)/day for 5 days, followed by the apheresis process to obtain a dose of at least 2×106CD34/kg. In this analysis, there was no bone marrow collection from any donor and no collection product was cryopreserved.
Support measuresDuring the aplasia, patients received transfusions to maintain platelets >20,000/μL, hemoglobin >8g/L%, and filgrastim 300μg/day until achieving neutrophil counts >500/μL. The preventive antimicrobial treatment consisted of levofloxacin 500mg daily, acyclovir 250mg EV, or 800mg orally every 12h, and fluconazole 400mg daily. Febrile neutropenia was treated according to the institutional guidelines. When fever presented, blood and urine culture and chest X-ray were performed immediately, and an antibiotic treatment was started with third-generation cephalosporins, aminoglycoside and vancomycin. With the persistence of fever for 72h, a computed tomography (CT) of paranasal cavities, thorax, abdomen, and pelvis was performed and blood fungal infection tests were performed. The adjustment of antimicrobial therapy was decided according to the radiological or blood test results. In the case of cytomegalovirus reactivation, preventive therapy with ganciclovir or foscarnet was initiated.
All patients received opioid analgesic treatment in case of grade 2–4 severe mucositis with parenteral nutrition, as needed.
Definitions of post-transplant complicationsThe GVHD was defined according to the time of appearance and development of the clinical characteristics. Thus, patients with GVHD in the first 100 days post-transplant were classified as acute GVHD and those with manifestations after that period were classified as chronic GVHD. Acute and chronic GVHD were graded using standard criteria.8,9 Overall survival was defined as the time elapsed between the day of the transplant and the date of death or the date of the last control. The progression-free survival was defined as the time elapsed between the day of the transplant and the date of relapse. Relapse was defined as evidence of recurrence of the underlying disease. Non-relapse mortality (NRM) was defined as any cause of death not associated with recurrence of the disease, including those associated with complications directly related to the transplant.
Statistical analysisThe Shapiro-Wilk test evaluated the normality of the quantitative variables. The demographic and baseline characteristics were presented using mean, percentage and ranges. The comparisons between the variables were made with the Chi-square method. The SG probabilities were estimated with the Kaplan–Meier method and the comparisons, with the log-rank test. The relapse, NRM, and GVHD analyses were performed in a competitive risk framework, using the non-parametric cumulative incidence estimator. The effect of the events that occurred during the follow-up and after transplantation, such as acute GVHD, was analyzed as a time-dependent covariate. The software SPSS.V.15 (IBM Software, USA) and Prisma Software V 6.0.1 (GraphPad Software, USA) were used for analysis. The differences were considered significant for values of p<0.05, with 95% confidence intervals (CIs).
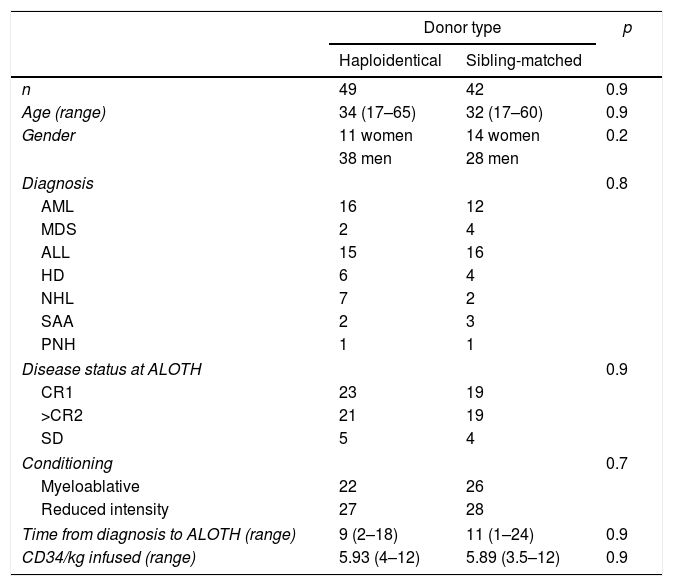
ResultsPatients, diseases, and characteristicsTable 1 shows the characteristics of the patients. During the analysis period, 91 patients were transplanted, 49 with HAPLO and 42 with SIB, with a mean age of 34 and 32 years, respectively, and a mean follow-up of 36 months (range 2–72 months). Two patients had anti-HLA antibodies specific to the donor (4%). A total of 40% of the SIB patients and 48% of the HAPLO patients had reactivation of the cytomegalovirus (CMV) (p=0.8), but there was no disease or mortality associated with this viral infection. In 8 patients, 4 in each group, the presence of polyomavirus (BK virus) was detected in the urine, which required adjustment of immunosuppression and symptomatic treatment, but none of the patients required the use of Cidofovir or renal replacement therapy.
Patient characteristics.
| Donor type | p | ||
|---|---|---|---|
| Haploidentical | Sibling-matched | ||
| n | 49 | 42 | 0.9 |
| Age (range) | 34 (17–65) | 32 (17–60) | 0.9 |
| Gender | 11 women | 14 women | 0.2 |
| 38 men | 28 men | ||
| Diagnosis | 0.8 | ||
| AML | 16 | 12 | |
| MDS | 2 | 4 | |
| ALL | 15 | 16 | |
| HD | 6 | 4 | |
| NHL | 7 | 2 | |
| SAA | 2 | 3 | |
| PNH | 1 | 1 | |
| Disease status at ALOTH | 0.9 | ||
| CR1 | 23 | 19 | |
| >CR2 | 21 | 19 | |
| SD | 5 | 4 | |
| Conditioning | 0.7 | ||
| Myeloablative | 22 | 26 | |
| Reduced intensity | 27 | 28 | |
| Time from diagnosis to ALOTH (range) | 9 (2–18) | 11 (1–24) | 0.9 |
| CD34/kg infused (range) | 5.93 (4–12) | 5.89 (3.5–12) | 0.9 |
AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; ALL: acute lymphoblastic leukemia; HD: Hodgkin's disease; NHL: non-Hodgkin lymphoma; SAA: severe aplastic anemia; PNH: paroxysmal nocturnal hemoglobinuria. CR1: first complete remission; CR2: second complete remission; SD: stable disease.
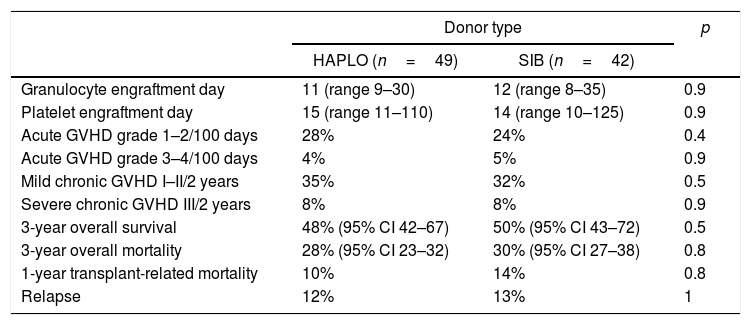
As shown in Table 2, there were no differences regarding the day of granulocyte or platelet graft arrest. The incidence at 30 days of hematopoietic recovery was 93% (95% CI 78–93) in HAPLO and 95% (95% CI 91–95) in SIB; p=0.9. The incidence of platelet recovery at 6 months was 88% (95% CI 79–93) and 93% (95% CI 91–94) in HAPLO and SIB (p=0.15), respectively.
Mortality unrelated to relapse and relapseNo significant differences were detected in transplant-related mortality, as shown in Tables 2 and 3. Similarly, mortality due to relapse did not differ significantly (HR 0.5814, CI 95% 0.2800–1.207). In two patients with a specific anti-donor antibody, it was necessary to perform desensitization; one survived with a normal functional graft and the other died due to sepsis associated with prolonged neutropenia.
Transplantation outcomes.
| Donor type | p | ||
|---|---|---|---|
| HAPLO (n=49) | SIB (n=42) | ||
| Granulocyte engraftment day | 11 (range 9–30) | 12 (range 8–35) | 0.9 |
| Platelet engraftment day | 15 (range 11–110) | 14 (range 10–125) | 0.9 |
| Acute GVHD grade 1–2/100 days | 28% | 24% | 0.4 |
| Acute GVHD grade 3–4/100 days | 4% | 5% | 0.9 |
| Mild chronic GVHD I–II/2 years | 35% | 32% | 0.5 |
| Severe chronic GVHD III/2 years | 8% | 8% | 0.9 |
| 3-year overall survival | 48% (95% CI 42–67) | 50% (95% CI 43–72) | 0.5 |
| 3-year overall mortality | 28% (95% CI 23–32) | 30% (95% CI 27–38) | 0.8 |
| 1-year transplant-related mortality | 10% | 14% | 0.8 |
| Relapse | 12% | 13% | 1 |
Mortality.
| Donor | p | ||
|---|---|---|---|
| HAPLO (n=13) | SIB (n=10) | ||
| Mortality not related to relapse | |||
| Myeloablative conditioning | 3 | 2 | 0.8 |
| Reduced intensity conditioning | 2 | 1 | 0.7 |
| Relapse | |||
| Myeloablative conditioning | 5 | 3 | 0.9 |
| Reduced intensity conditioning | 3 | 3 | 0.9 |
| Graft-versus-host disease | 0 | 0 | |
| Other causes after 2 years of ALOTH | 0 | 1 | n.s. |
As shown in Tables 2 and 3, the incidence of mild (grades 1 and 2) and severe (grades 3 and 4) acute and chronic GVHD was not different in the two groups of patients. Cumulative incidence adjusted by competitive risks for global acute and chronic in HAPLO was 34% and 43%, respectively, and for SIB, 41% and 29%, respectively (p=0.9%).
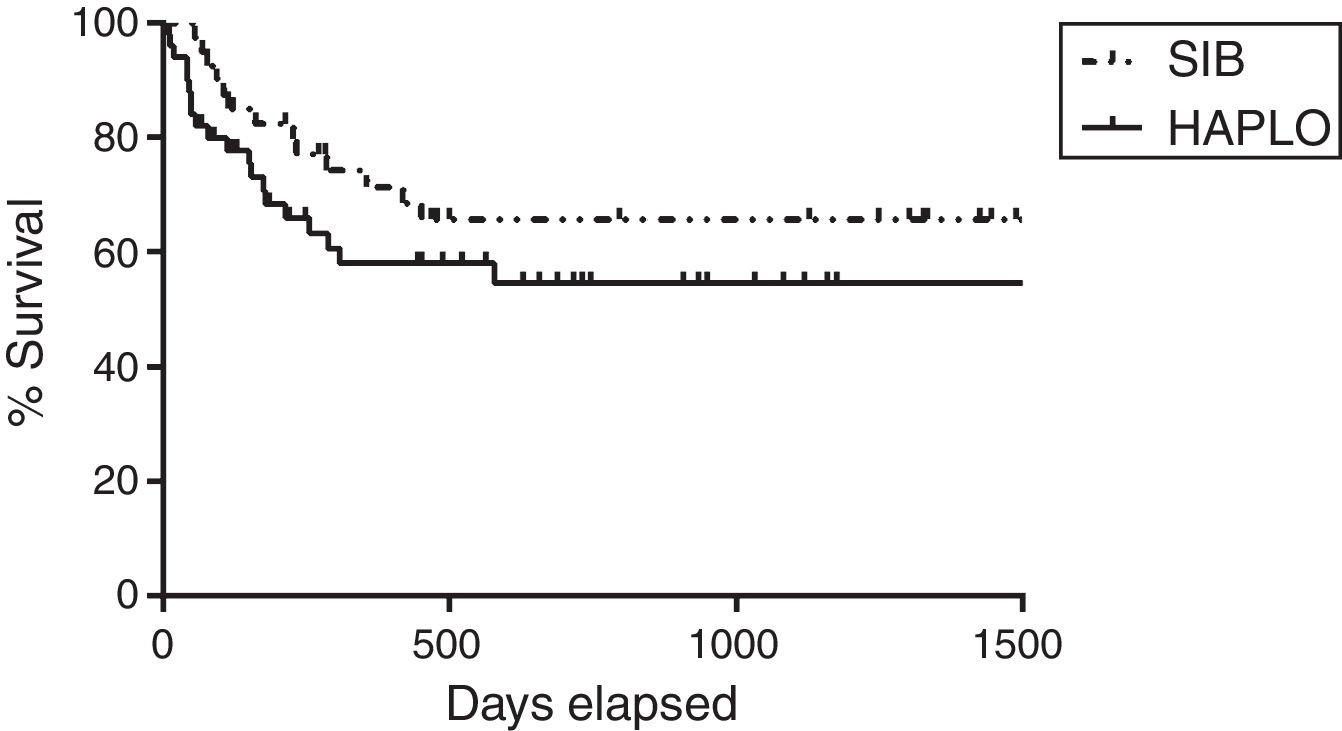
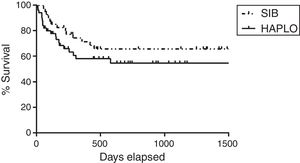
Overall survivalAs shown in Table 2 and Figure 1, the overall survival estimated at 3 years in HAPLO was 48%, 95% CI 42–67, and in SIB, 50%, 95% CI 43–72 (p=0.5), without significant differences in the two groups when adjusting the analysis by incidence of GVHD, relapse, or conditioning intensity. Table 3 shows global survival and disease-free survival analysis, adjusted by conditioning and GVHD, but no significant differences were observed.
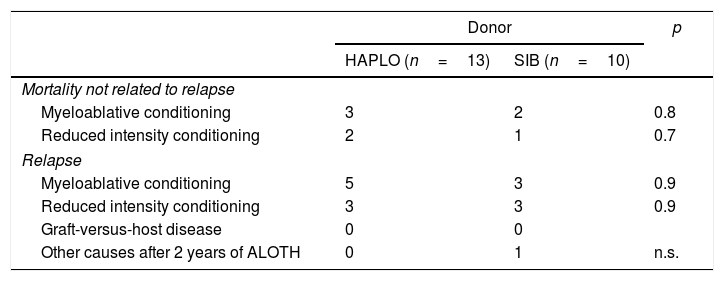
MortalityAs shown in Table 3, there were no significant differences among the patient causes of death. Thus, 13 patients in the HAPLO group died, 61% due to relapse and 39% due to mortality related to transplantation; 11 patients died in the SIB group, 60% due to relapse, 35% due to mortality related to transplantation and 5% due to other causes. Patients who died due to HAPLO transplant-related mortality were by sepsis3 and cerebral hemorrhage,1 and in the SIB group, by sepsis2 and multiorgan failure.1
DiscussionThe ALOTH is curative for several hematological diseases. The HLA identical related donor is the donor of choice,10 but not all patients have access. The HAPLO has emerged as a good strategy with results similar to those obtained with SIB.11 However, this approach is not new. For more than 6 decades, the ALOTH HAPLO has been used for primary immunodeficiencies with success,12,13 but when applying this modality in patients with leukemia, the results were not encouraging.14 The major change, in which megadoses of hematopoietic precursors with T-lymphocyte depletion were used, which led to an exponential increase in the use of the ALOTH HAPLO, was made by the Peruggia group,15–17 followed by various North American groups, thus achieving adequate engraftment and GVHD rates in a tolerable range. Some of the issues related to the T-cell depleted modalities were elevated costs, ex vivo manipulation of the graft, and the elimination of regulatory T lymphocytes, which is necessary for the graft vs leukemia effect. A major breakthrough was later reported by the Baltimore group, who showed that HAPLO without T-cell depletion with post-transplant cyclophosphamide achieved adequate engraftment and prolonged disease-free survival rates. This modality is economical, widely available, and presents tolerable toxicity.18 Since then, several groups have reported comparable results between the HAPLO and SIB. For developing countries, the HAPLO has become an excellent resource for transplantation, as the acquisition of umbilical cord or unrelated donors is expensive and presents marginal access. In our program, since 2012, we have adopted the HAPLO modality without ex vivo depletion of T cells, with post-transplant cyclophosphamide, as a source of alternative precursors. Our data confirm that the ALOTH, either with SIB or with HAPLO, has comparable results. Our experience has allowed us to clarify that the implementation of the ALOTH HAPLO requires the same logistic and patient care process as the ALOTH SIB. Remarkably, because the option of the HAPLO has become available, the number of patients transplanted each year has increased considerably, directly reflecting on the improvement in access, as well as showing family structural changes, as families increasingly have fewer descendants. The possible criticisms of our study derive from it being a retrospective analysis; however, studies in a real scenario, away from the stringent control of the root cause analysis (RCT), are valuable. In the HAPLO setting, the detection of anti-HLA antibodies has become a priority, as their presence induces high rates of graft failure and mortality. In our program, we detected two patients with this condition, who underwent the desensitization protocol that resulted in a successful transplant in one of them.17 Without any doubt, this situation deserves further analysis in the future. Our analyses allow us to conclude that the performance of haploidentical transplants in adults with either myeloablative or reduced intensity scheme allow for the acquisition of results similar to those obtained with identical HLA donors and can be performed in our country.
Conflicts of interestThe authors declare no conflicts of interest.