Cerebrovascular disease, particularly stroke, is one of the most severe clinical complications associated with sickle cell disease and is a significant cause of morbidity in both children and adults. Over the past two decades, considerable advances have been made in the understanding of its natural history and enabled early identification and treatment of children at the highest risk. Transcranial Doppler screening and regular blood transfusions have markedly reduced the risk of stroke in children. However, transcranial Doppler has a limited positive predictive value and the pathophysiology of cerebrovascular disease is not completely understood. In this review, we will focus on the current state of knowledge about risk factors associated with ischemic stroke in patients with sickle cell disease. A search of PubMed was performed to identify studies. Full texts of the included articles were reviewed and data were summarized in a table. The coinheritance of alpha-thalassemia plays a protective role against ischemic stroke. The influence of other genetic risk factors is controversial, still preliminary, and requires confirmatory studies. Recent advances have established the reticulocyte count as the most important laboratory risk factor. Clinical features associated with acute hypoxemia as well as silent infarcts seem to influence the development of strokes in children. However, transcranial Doppler remains the only available clinical prognostic tool to have been validated. If our understanding of the many risk factors associated with stroke advances further, it may be possible to develop useful tools to detect patients at the highest risk early, improving the selection of children requiring intensification therapy.
Sickle cell disease (SCD) is a group of autosomal recessive genetic disorders characterized by the presence of at least one βS allele (HBB:c.20A→T) of the HBB gene that encodes the beta chain of hemoglobin (Hb).1,2 The translation of a βS allele generates Hb S which results from the substitution of a normal hydrophilic amino acid (glutamic acid) by a hydrophobic amino acid (valine) at position six in the variant beta globin chain. As the valine residue interacts with adjacent complementary sites of globin chains, the resulting protein is prone to polymerization.3,4
In certain situations such as hypoxia, acidosis, and dehydration, Hb S molecules form elongated polymers that modify the cytoskeleton of red blood cells (RBCs), originating the characteristic ‘sickle’ shape (sickling). Polymerization of Hb S causes several physical and chemical changes in RBCs, and is the primary event essential for the pathogenesis of SCD.4,5 When a critical concentration of the Hb S polymer is reached in RBCs, cell damage occurs and, consequently, the phenotypic manifestations of SCD, characterized by chronic severe hemolytic anemia and vaso-occlusion, arise.6
Cerebrovascular disease (CVD) is one of the most severe complications of SCD, affecting about 50% of individuals by 14 years of age.7 Without early therapeutic intervention, overt ischemic stroke (hereafter, stroke), the most severe type of CVD, occurs in about 11% of individuals before 20 years of age.8 The natural history of stroke in SCD is well described9,10; however, its pathophysiology is not fully understood.11 Few risk factors are established8 except for the increased cerebral blood flow in the arteries of the Willis circle detected by transcranial Doppler ultrasonography (TCD).12
Although TCD is recognized as a sensitive predictor of stroke risk, the specificity of the technique is relatively low, and the positive predictive value is low. About 60% of individuals at high-risk of stroke detected by TCD will not have a stroke13 and it is unnecessary to subject them to prophylactic blood transfusions12 or hydroxyurea therapy.14
There is no available method to predict which children with high-risk TCD will not have a stroke and thus would not benefit from prophylactic blood transfusions or hydroxyurea therapy. Recent data from Nigeria showed that none out of 17 children who had high-risk TCD and whose parents or guardians had refused a prophylactic blood transfusion program developed a stroke in a mean follow-up of 27.3±11.1 months.15 Only about 10% of individuals who had high-risk TCD will suffer from stroke within one year after the confirmatory test.16 Furthermore, it is estimated that to prevent the occurrence of an episode of stroke, it would be necessary to put seven children into the prophylactic blood transfusion program.17
Stroke still occurs in children with normal TCD.13,16 There is a relatively large variability in blood flow velocities in the same children examined at regular intervals.18 Furthermore, access to TCD screening and to prophylactic blood transfusion programs is often absent or limited,19,20 especially in developing countries.20,21 Additionally, TCD screening programs have poor adherence all over the world,19,21–25 and, in some services, a TCD screening program is not available at all.
Prophylactic blood transfusion programs have several side effects, such as transfusion-transmitted infections, alloimmunization, and iron overload, among others. We emphasize the high prevalence of alloimmunization. Recently, data from Philadelphia showed that 57.7% of individuals with SCD in prophylactic blood transfusion programs become alloimmunized despite transfusion from Rh-matched minority donors.26 The risk of iron overload and the high cost of chelation therapy also deserve a mention when evaluating the disadvantages of a prophylactic blood transfusion program.27 There are no data about the effect of prophylactic blood transfusion and iron overload on mortality in individuals with SCD.28 Some families and hematologists refuse long-term transfusion therapy. The reasons for refusing a prophylactic blood transfusion program are diverse, and include the high cost of treatment, unavailability of blood, and the unlimited duration of the program.15
Due to the phenotypic heterogeneity of SCD, there is interest in predicting which individuals would be most severely affected. However, physicians are still unable to certainly predict which children will have clinically more severe disease during childhood.29 As mentioned before, early identification of children at the highest risk of developing a stroke would allow early interventions such as a prophylactic blood transfusion program,12 hydroxyurea therapy,14 or bone marrow transplantation,30 before the development of motor and/or neurocognitive sequelae. Conversely, more accurate risk prediction would avoid the indication of risky and potentially toxic therapies in individuals with low risk. Moreover, it would be possible to avoid the considerable increase in the costs of treatment and management of individuals with stroke. The cost of prophylactic blood transfusion programs has been estimated at US$40,000 per year with deferoxamin,31 and €45,000 per year with deferasirox.7 Additionally, a stroke event requires additional rehabilitation costs of US$40,000 per year.32 Also, it would be possible to reduce the incidence, morbidity, and mortality derived from stroke and, consequently, to improve the life expectancy and quality of life in children with SCD.
Several studies have been conducted to identify risk factors associated with CVD in individuals with SCD. In this review, we identify and compile data about genetic, laboratory and clinical risk factors associated with the development of stroke in individuals with SCD.
MethodsArticles indexed with the following search terms and combinations of them were retrieved from PubMed: ‘sickle cell disease’, ‘sickle cell anemia’, ‘stroke’, ‘cerebrovascular disease’, ‘risk factors’ and ‘polymorphism’. There were no restrictions on date or language of publication. The titles and abstracts of the articles were evaluated. Articles considered outside the scope of this review were excluded. The full texts of all potential articles were read in detail. If deemed relevant by the authors, the data were extracted from the articles and were compiled in Table 1. We also included relevant articles that had been listed in the references of the articles found using the strategy described above. Review articles were cited to provide readers with more details and references about the topics covered by the review.
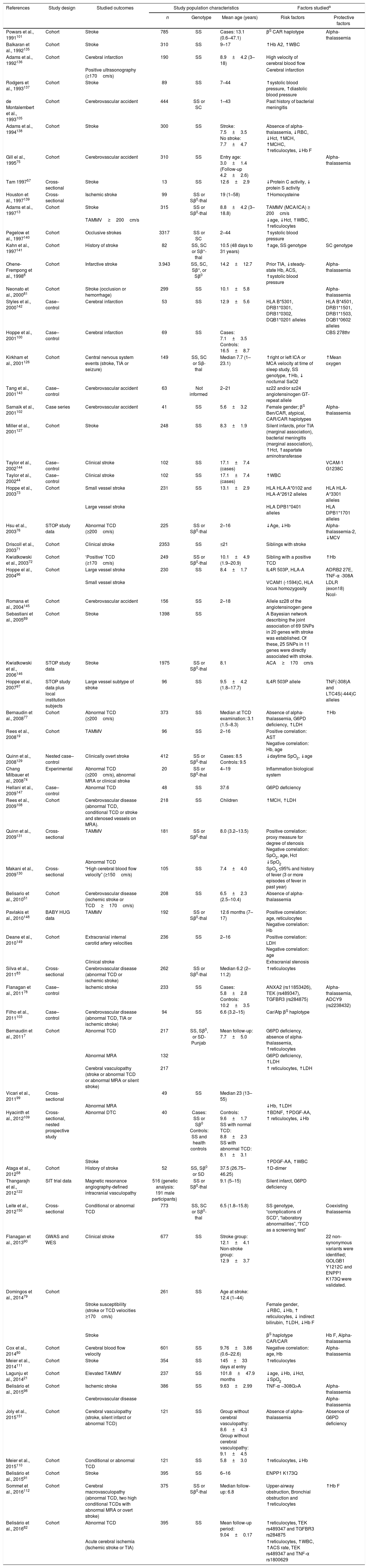
Published factors reported to contribute to the risk of stroke in individuals with sickle cell disease.
| References | Study design | Studied outcomes | Study population characteristics | Factors studieda | |||
|---|---|---|---|---|---|---|---|
| n | Genotype | Mean age (years) | Risk factors | Protective factors | |||
| Powars et al., 1991101 | Cohort | Stroke | 785 | SS | Cases: 13.1 (0.6–47.1) | βS CAR haplotype | Alpha-thalassemia |
| Balkaran et al., 1992135 | Cohort | Stroke | 310 | SS | 9–17 | ↑Hb A2, ↑WBC | |
| Adams et al., 1992136 | Cohort | Cerebral infarction | 190 | SS | 8.9±4.2 (3–18) | High velocity of cerebral blood flow | |
| Positive ultrasonography (≥170cm/s) | Cerebral infarction | ||||||
| Rodgers et al., 1993137 | Cohort | Stroke | 89 | SS | 7–44 | ↑systolic blood pressure, ↑diastolic blood pressure | |
| de Montalembert et al., 1993105 | Cohort | Cerebrovascular accident | 444 | SS or SC | 1–43 | Past history of bacterial meningitis | |
| Adams et al., 1994138 | Cohort | Stroke | 300 | SS | Stroke: 7.5±3.5 No stroke: 7.7±4.7 | Absence of alpha-thalassemia, ↓RBC, ↓Hct, ↑MCH, ↑MCHC, ↑reticulocytes, ↓Hb F | |
| Gill el al., 199575 | Cohort | Cerebrovascular accident | 310 | SS | Entry age: 3.0±1.4 (Follow-up 4.2±2.6) | Alpha-thalassemia | |
| Tam 199757 | Cross-sectional | Stroke | 13 | SS | 12.6±2.9 | ↓Protein C activity, ↓ protein S activity | |
| Houston et al., 1997139 | Cross-sectional | Ischemic stroke | 99 | SS or Sβ0-thal | 19 (1–58) | ↑Homocysteine | |
| Adams et al., 199713 | Cohort | Stroke | 315 | SS or Sβ0-thal | 8.8±4.2 (3–18.8) | TAMMV (MCA/ICA) ≥ 200cm/s | |
| TAMMV≥200cm/s | ↓age, ↓Hct, ↑WBC, ↑reticulocytes | ||||||
| Pegelow et al., 1997140 | Cohort | Occlusive strokes | 3317 | SS or SC | 2–44 | ↑systolic blood pressure | |
| Kahn et al., 1997141 | Cohort | History of stroke | 82 | SS, SC or Sβ+-thal | 10.5 (48 days to 31 years) | ↑age, SS genotype | SC genotype |
| Ohene-Frempong et al., 19988 | Cohort | Infarctive stroke | 3.943 | SS, SC, Sβ+, or Sβ0 | 14.2±12.7 | Prior TIA, ↓steady-state Hb, ACS, ↑systolic blood pressure | Alpha-thalassemia |
| Neonato et al., 200081 | Cohort | Stroke (occlusion or hemorrhage) | 299 | SS | 10.1±5.8 | Alpha-thalassemia | |
| Styles et al., 2000142 | Case–control | Cerebral infarction | 53 | SS | 12.9±5.6 | HLA B*5301, DRB1*0301, DRB1*0302, DQB1*0201 alleles | HLA B*4501, DRB1*1501, DRB1*1503, DQB1*0602 alleles |
| Hoppe et al., 2001100 | Case–control | Cerebral infarction | 69 | SS | Cases: 7.1±3.5 Controls: 16.5±8.7 | CBS 278thr | |
| Kirkham et al., 2001126 | Cohort | Central nervous system events (stroke, TIA or seizure) | 149 | SS, SC or Sβ-thal | Median 7.7 (1–23.1) | ↑right or left ICA or MCA velocity at time of sleep study, SS genotype, ↑Hb, ↓ nocturnal SaO2 | ↑Mean oxygen |
| Tang et al., 2001143 | Case–control | Cerebrovascular accident | 63 | Not informed | 2–21 | sz22 and/or sz24 angiotensinogen GT-repeat allele | |
| Sarnaik et al., 2001102 | Case series | Cerebrovascular accident | 41 | SS | 5.6±3.2 | Female gender; βS Ben/CAR, atypical, CAR/CAR haplotypes | Alpha-thalassemia |
| Miller et al., 2001127 | Cohort | Stroke | 248 | SS | 8.3±1.9 | Silent infarcts, prior TIA (marginal association), bacterial meningitis (marginal association), ↑Hct, ↑aspartate aminotransferase | |
| Taylor et al., 2002144 | Case–control | Clinical stroke | 102 | SS | 17.1±7.4 (cases) | VCAM-1 G1238C | |
| Taylor et al., 200244 | Case–control | Clinical stroke | 102 | SS | 17.1±7.4 (cases) | ↑WBC | |
| Hoppe et al., 200373 | Cohort | Small vessel stroke | 231 | SS | 13.1±2.9 | HLA HLA-A*0102 and HLA-A*2612 alleles | HLA HLA-A*3301 alleles |
| Large vessel stroke | HLA DPB1*0401 alleles | HLA DPB1*1701 alleles | |||||
| Hsu et al., 200376 | STOP study data | Abnormal TCD (≥200cm/s) | 225 | SS or Sβ0-thal | 2–16 | ↓Age, ↓Hb | Alpha-thalassemia-2, ↓MCV |
| Driscoll et al., 200371 | Cohort | Clinical stroke | 2353 | SS | ≤21 | Siblings with stroke | |
| Kwiatkowski et al., 200372 | Cohort | ‘Positive’ TCD (≥170cm/s) | 249 | SS or Sβ0-thal | 10.1±4.9 (1.9–20.9) | Sibling with a positive TCD | ↑Hb |
| Hoppe el al., 200496 | Cohort | Large vessel stroke | 230 | SS | 8.4±1.7 | IL4R 503P, HLA-A | ADRB2 27E, TNF-α -308A |
| Small vessel stroke | VCAM1 (-1594)C, HLA locus homozygosity | LDLR (exon18) Ncol- | |||||
| Romana et al., 2004145 | Cohort | Cerebrovascular accident | 156 | SS | 2–18 | Allele sz28 of the angiotensinogen gene | |
| Sebastiani et al., 200589 | Cohort | Stroke | 1398 | SS | A Bayesian network describing the joint association of 69 SNPs in 20 genes with stroke was established. Of these, 25 SNPs in 11 genes were directly associated with stroke. | ||
| Kwiatkowski et al., 2006146 | STOP study data | Stroke | 1975 | SS or Sβ0-thal | 8.1 | ACA≥170cm/s | |
| Hoppe et al., 200797 | STOP study data plus local institution subjects | Large vessel subtype of stroke | 96 | SS | 9.5±4.2 (1.8–17.7) | IL4R 503P allele | TNF(-308)A and LTC4S(-444)C alleles |
| Bernaudin et al., 200877 | Cohort | Abnormal TCD (≥200cm/s) | 373 | SS | Median at TCD examination: 3.1 (1.5–8.3) | Absence of alpha-thalassemia, G6PD deficiency, ↑LDH | ↑Hb |
| Rees et al., 200819 | Cohort | TAMMV | 96 | SS | 2–16 | Positive correlation: AST Negative correlation: Hb, age | |
| Quinn et al., 2008129 | Nested case–control | Clinically overt stroke | 412 | SS or Sβ0-thal | Cases: 8.5 Controls: 9.5 | ↓daytime SpO2, ↓age | |
| Chang Milbauer et al., 200874 | Experimental | Abnormal TCD (≥200cm/s), abnormal MRA or clinical stroke | 20 | SS or Sβ0-thal | 4–19 | Inflammation biological system | |
| Hellani et al., 2009147 | Case–control | Abnormal TCD | 48 | SS | 37.6 | G6PD deficiency | |
| Rees et al., 2009108 | Cohort | Cerebrovascular disease (abnormal TCD, conditional TCD or stroke and stenosed vessels on MRA). | 218 | SS | Children | ↑MCH, ↑LDH | |
| Quinn et al., 2009131 | Cross-sectional | TAMMV | 181 | SS or Sβ0-thal | 8.0 (3.2–13.5) | Positive correlation: proxy measure for degree of stenosis Negative correlation: SpO2, age, Hct | |
| Abnormal TCD | ↓SpO2 | ||||||
| Makani et al., 2009130 | Cross-sectional | “High cerebral blood flow velocity” (≥150cm/s) | 105 | SS | 7.4±4.0 | SpO2 ≤95% and history of fever (3 or more episodes of fever in past year) | |
| Belisario et al., 201051 | Cohort | Cerebrovascular disease (ischemic stroke or TCD≥170cm/s) | 208 | SS | 6.5±2.3 (2.5–10.4) | Absence of alpha-thalassemia | |
| Pavlakis et al., 2010148 | BABY HUG data | TAMMV | 192 | SS or Sβ0-thal | 12.6 months (7–17) | Positive correlation: age, reticulocytes Negative correlation: Hb | |
| Deane et al., 2010149 | Cohort | Extracranial internal carotid artery velocities | 236 | SS | 2–16 | Positive correlation: LDH Negative correlation: age | |
| Clinical stroke | Extracranial stenosis | ||||||
| Silva et al., 201183 | Cross-sectional | Cerebrovascular disease (abnormal TCD or ischemic stroke) | 262 | SS or Sβ0-thal | Median 6.2 (2–11.2) | ↑reticulocytes | |
| Flanagan et al., 201178 | Case–control | Ischemic stroke | 233 | SS | Cases: 5.8±2.8 Controls: 10.2±3.5 | ANXA2 (rs11853426), TEK (rs489347), TGFBR3 (rs284875) | Alpha-thalassemia, ADCY9 (rs2238432) |
| Filho et al., 2011103 | Case–control | Cerebrovascular disease (abnormal TCD, TIA or ischemic stroke) | 94 | SS | 6.6 (3.2–15) | Car/Atp βS haplotype | |
| Bernaudin et al., 20117 | Cohort | Abnormal TCD | 217 | SS, Sβ0, or SD-Punjab | Mean follow-up: 7.7±5.0 | G6PD deficiency, absence of alpha-thalassemia, ↑reticulocytes | |
| Abnormal MRA | 132 | G6PD deficiency, ↑LDH | |||||
| Cerebral vasculopathy (stroke or abnormal TCD or abnormal MRA or silent stroke) | 217 | ↑ reticulocytes, ↑LDH | |||||
| Vicari et al., 201199 | Cross-sectional | 49 | SS | Median 23 (13–55) | |||
| Abnormal MRA | ↓Hb, ↑LDH | ||||||
| Hyacinth et al., 2012109 | Cross-sectional, nested prospective study | Abnormal DTC | 40 | Cases: SS or Sβ0 Controls: SS and health controls | Controls: 9.6±1.7 SS with normal TCD: 8.8±2.3 SS with abnormal TCD: 8.1±3.1 | ↑BDNF, ↑PDGF-AA, ↑ reticulocytes, ↓Hb | |
| Stroke | ↑PDGF-AA, ↑WBC | ||||||
| Ataga et al., 201258 | Cohort | History of stroke | 52 | SS, Sβ0 or SD | 37.5 (26.75–46.25) | ↑D-dimer | |
| Thangarajh et al., 2012122 | SIT trial data | Magnetic resonance angiography-defined intracranial vasculopathy | 516 (genetic analysis: 191 male participants) | SS or Sβ0-thal | 9.1 (5–15) | Silent infarct, G6PD deficiency | |
| Leite et al., 2012150 | Cross-sectional | Conditional or abnormal TCD | 773 | SS, SC or Sβ0-thal | 6.5 (1.8–15.8) | SS genotype, “complications of SCD”, “laboratory abnormalities”, “TCD as a screening test” | Coexisting thalassemia |
| Flanagan et al., 201390 | GWAS and WES | Clinical stroke | 677 | SS | Stroke group: 12.1±4.1 Non-stroke group: 12.9±3.7 | 22 non-synonymous variants were identified; GOLGB1 Y1212C and ENPP1 K173Q were validated. | |
| Domingos et al., 201479 | Cohort | 261 | SS | Age at stroke: 12.4 (1–44) | |||
| Stroke susceptibility (stroke or TCD velocities ≥170cm/s) | Female gender, ↓RBC, ↓Hb, ↑ reticulocytes, ↓ indirect bilirubin, ↑LDH, ↓Hb F | ||||||
| Stroke | βS haplotype CAR/CAR | Hb F, Alpha-thalassemia | |||||
| Cox et al., 201480 | Cohort | Cerebral blood flow velocity | 601 | SS | 9.76±3.86 (0.6–22.6) | Negative correlation: age, Hb | Alpha-thalassemia |
| Meier et al., 2014111 | Cohort | Stroke | 354 | SS | 145±33 days at entry | ↑reticulocytes | |
| Lagunju et al., 201421 | Cohort | Elevated TAMMV | 237 | SS | 101.8±47.9 months | ↓age, ↓Hb, ↓Hct, ↓SpO2 | |
| Belisário et al., 201598 | Cohort | Ischemic stroke | 386 | SS | 9.63±2.99 | TNF-α −308G>A | Alpha-thalassemia |
| Cerebrovascular disease | Alpha-thalassemia | ||||||
| Joly et al., 2015151 | Cohort | Cerebral vasculopathy (stroke, silent infarct or abnormal TCD) | 121 | SS | Group without cerebral vasculopathy: 8.6±4.3 Group without cerebral vasculopathy: 9.1±4.5 | Absence of alpha-thalassemia | Absence of G6PD deficiency |
| Meier et al., 2015110 | Cohort | Conditional or abnormal TCD | 121 | SS | 5.8±3.0 | ↑reticulocytes, ↓Hb | |
| Belisário et al., 201591 | Cohort | Stroke | 395 | SS | 6–16 | ENPP1 K173Q | |
| Sommet et al., 2016112 | Cohort | Cerebral macrovasculopathy (abnormal TCD, two high conditional TCDs with abnormal MRA or overt stroke) | 375 | SS or Sβ0-thal | Median follow-up: 6.8 | Upper-airway obstruction, Bronchial obstruction and ↑reticulocytes | ↑Hb F |
| Belisário et al., 201682 | Cohort | Abnormal TCD | 395 | SS | Mean follow-up period: 9.04±0.17 | ↑reticulocytes, TEK rs489347 and TGFBR3 rs284875 | |
| Acute cerebral ischemia (Ischemic stroke or TIA) | ↑reticulocytes, ↑WBC, ↑ACS rate, TEK rs489347 and TNF-α rs1800629 | ||||||
The effects showed in the table were those described by the authors of the papers; possible methodological biases were not taken into account. The heterogeneity between populations of individuals with sickle cell disease, the age of the patients, and the number of individuals in each study may lead to controversial interpretation of results. When both univariate and multivariate analysis were presented in the studies, only results of multivariate analysis were considered.
ACA: anterior cerebral artery; ACS: acute chest syndrome; ADCY9: adenylate cyclase 9; ADRB: beta adrenergic receptor; ANXA2: annexin A2; AST: aspartate aminotransferase; Atp: atypical; BDNF: brain derived neurotropic factor; Ben: Benin; CAR: Central African Republic; CBS: cystathionine β-synthase; ENPP1: ectonucleotide pyrophosphatase/phosphodiesterase 1; GOLGB1: golgin B1; GWAS: genome-wide association study; Hb: hemoglobin; Hct: hematocrit; HLA: human leukocyte antigen; ICA: internal carotid artery; IL4R: interleukin-4 receptor; LDH: lactate dehydrogenase; LDLR: low density lipoprotein receptor; MCA: middle cerebral artery; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MRA: magnetic resonance angiogram; PDGF: platelet derived growth factor; SpO2: blood oxygen saturation; STOP: Stroke Prevention Trial in Sickle Cell Disease; TAMMV: Time averaged maximum mean velocity; TCD: transcranial Doppler; TEK: tyrosine kinase; TGFBR3: transforming growth factor beta receptor 3; TIA: transient ischemic attack; TNF-α: tumor necrosis factor alpha; WBC: white blood cell; and WES: whole-exome sequencing.
The pathophysiology of stroke in individuals with SCD involves multiple mechanisms.33 The chronology and hierarchy, however, are not well established.34 The two main mechanisms responsible for stroke in individuals affected by the disease are: (1) occlusive vasculopathy characterized by the proliferation of smooth muscle cells and increased fibroblasts in the intima layer of artery walls and, consequently, progressive segmental narrowing of the distal internal carotid artery and proximal branches of the main intracranial arteries (circle of Willis) and (2) sickled RBC aggregation, and consequent occlusion of small vessel lumen.35 Some previously proposed models11,33,35,36 provide an overview of the pathophysiology of cerebral vasculopathy in SCD.
Sickled RBCs firmly adhere to the vascular endothelium of intramural small vessels of the arteries of Willis circle through several RBC-endothelial bridges.37,38 It is believed that the triggering factor in the pathogenesis of stroke in individuals with SCD is the adhesion of sickled RBCs and/or reticulocytes to the vascular endothelium, generating endothelial activation and damage.36 High expressions of endothelial adhesion molecules and RBC adhesion molecules have been observed in individuals with SCD, including integrins,39,40 endothelial selectins,41,42 soluble adhesion molecules,43 and immunoglobulin superfamily members.44 The adhesion of RBCs in the microvasculature causes the entrapment of denser and less deformable cells, decreasing the blood flow, and increasing the capillary transit time.45 This favors further polymerization of Hb S and causes vaso-occlusion.
Adhesion molecules, cytokines, and chemoattractants attract white blood cells (WBCs) to the site of damaged endothelium, causing microvascular obstruction and ischemia.46,47 The activated state of WBCs caused by the chronic inflammatory state characteristic of SCD causes a significant number of WBCs exhibiting high levels of molecules that can bind to the endothelium. Abnormal adhesion of RBCs and WBCs occurs mainly in the post-capillary venules where shear stress is sufficiently low to allow blood cell adhesion to the vascular wall. Cell adhesion to endothelium may also occur in larger arteries. However, it is improbable that the abnormal adhesion of RBCs and WBCs to the endothelium occurs in large cerebral arteries of individuals with SCD, resulting in stroke.11 Alternatively, abnormal adhesion happens in the venules of large arteries and the pathophysiological process occurs from the wall into the lumen of large arteries.
The role of hemolysis is evident in the pathophysiology of CVD.11 Chronic and acute hemolysis results in free hemoglobin that interferes with the nitric oxide (NO) metabolism. The release of arginase derived from hemolysis consumes l-arginine, a substrate for the production of NO. Free Hb, heme, and heme iron catalyze the production of oxygen radicals, potential NO scavengers and endothelium activators.48 The reduction in NO bioavailability and increase of oxygen free radical formation lead to endothelial dysfunction, increasing inflammation and contributing to a hypercoagulable state associated to SCD. Furthermore, reduced NO bioavailability limits smooth muscle relaxation and increases vascular resistance.11,49
Reduced NO bioavailability reduces vasodilation and impairs the inhibition of platelet activation and aggregation mediated by NO, and also inhibits the repression of cell adhesion molecule transcription.49 The role of vascular tone regulation on the pathophysiology of stroke is not clear, but there is evidence supporting its involvement, such as the reduction of plasma free Hb levels and hemolysis secondary to chronic transfusion therapy.50 The co-inheritance of alpha-thalassemia also decreases hemolysis51 and preserves the benefit of higher NO bioavailability.34
Adherent platelets aggregate at the site of endothelial injury, forming a web of cells together with WBCs and RBCs.33,36,52–54 Coagulation system abnormalities have been reported in individuals with SCD, generating a hypercoagulable state. Coagulation and fibrinolysis markers are elevated at steady state and are more elevated during vaso-occlusive crises.55,56 Low concentrations of protein C and S were associated with history of stroke,57 as well as high concentrations of the D-dimer.58 However, the relationship of coagulation and fibrinolysis markers with stroke has not been fully elucidated.
In response to chronic severe anemia, cerebral blood flow and cerebral blood velocity are increased in individuals with SCD.59,60 Any decrease in cerebral blood flow by physiological or pathological reasons leads to a risk of imbalance between demand and supply of oxygen in the brain, increasing the risk of stroke.11 Increased hypoxia and the inability of the brain vasculature to dilate lead to ischemia.61 Furthermore, hypoxia and ischemia may increase the expression of several adhesion molecule receptors in the vascular endothelium of the human brain. Increased transcription of several genes involved in angiogenesis, inflammation, vascular tone regulation, cell proliferation, apoptosis, and coagulation in hypoxic situations could contribute to cerebral vasculopathy.11
After temporary hypoxia followed by re-oxygenation in order to induce reversible sickling, transgenic mice exhibit an excessive inflammatory response characterized by increased WBC adhesion and extravasation in the microvasculature, and evidence of oxidant production in the vascular endothelium.62 This pro-inflammatory state leads to intimal hyperplasia and proliferation of smooth muscle cells and fibroblasts, progressive stenosis of the affected intracranial artery and, finally, occlusion.63,64
Diagnosis of stroke riskTranscranial Doppler ultrasound measures the blood flow velocity in the brain arteries and is an important tool to detect the risk of stroke in children with SCD. Results of Transcranial Doppler assist physicians to include high-risk individuals on chronic transfusion or hydroxyurea therapy to prevent the occurrence of the first stroke (primary prevention).12,14,30
The Stroke Prevention Trial in Sickle Cell Anemia (STOP), a randomized clinical trial performed in children with SCD and high-risk of stroke detected by TCD [time averaged maximum mean velocity (TAMMV) in the internal carotid or middle cerebral artery ≥200cm/s], showed a 92% decreased difference in risk of the first stroke in the group treated with chronic transfusion therapy compared with the standard treatment (observational). Children with TAMMV ≥200cm/s have a 10% risk per year of developing stroke, which may be reduced to less than 1% with chronic transfusion therapy.12 The STOP study was halted earlier than planned after this clear-cut evidence was found. Based on these results, the National Institutes of Health (NIH) recommended TCD screening of children with SCA to assess the risk of stroke development, and chronic transfusion therapy to reduce risk of stroke in children at high risk. Consequently, there was a 45% reduction in the incidence of hospitalizations due to stroke in the USA, as well as a 45% reduction of hospital stays and 24% decrease in hospital fees attributable to stroke, when comparing the pre- and post-STOP published data.65
After the publication of STOP, several studies have been released reporting reduction of stroke incidence in children with SCD.7,66–69 Data from the Centre Hospitalier Intercommunal in France showed that TCD and intensification therapy (chronic transfusion therapy, bone marrow transplantation, or hydroxyurea therapy) reduced the cumulative risk of stroke before 18 years of age from 11% to 1.9%.7
The STOP-2 trial evaluated the possibility of interrupting chronic transfusion therapy after TAMMV normalization in children with SCA and high risk of stroke development detected by TCD. STOP-2 was also halted early after some evidence that discontinuing chronic transfusion therapy results in reversion to high-risk TCD or stroke in many children.70
Recently, the NIH also halted early the Transcranial Doppler with Transfusions Changing to Hydroxyurea (TWiTCH) trial. The aim of the study was to evaluate whether hydroxyurea therapy would lower the TCD TAMMV in children with SCD to a similar degree as chronic transfusion therapy. Children on chronic transfusion therapy to prevent the first stroke (at least one year; mean time of 4.6 years) were randomized to keep receiving transfusions or discontinuing chronic transfusion therapy and initiating hydroxyurea therapy. Hydroxyurea therapy was not inferior to chronic transfusion therapy in reducing TCD TAMMV in children with SCD at high-risk for stroke development and no magnetic resonance angiography (MRA)-defined severe vasculopathy at the entry of the study.14
Genetic, laboratory, and clinical risk factors for the development of cerebrovascular disease and strokeSeveral studies have been conducted to establish risk factors for the development of stroke in individuals with SCD. However, the association of the underlying vasculopathy of stroke with these risk factors is not well set. Table 1 summarizes characteristics and results of studies evaluating the influence of genetic, laboratory, and clinical factors on the occurrence of stroke in individuals with SCD. Data from these studies suggest that certain characteristics are mainly responsible for the occurrence of stroke in SCD (Table 2). Studies that evaluated some risk factors with no significant association with stroke were not included in Table 1 due to the limited space. Studies aimed to evaluate risk factors for silent infarcts and hemorrhagic stroke alone were not included because they were out of the scope of this review.
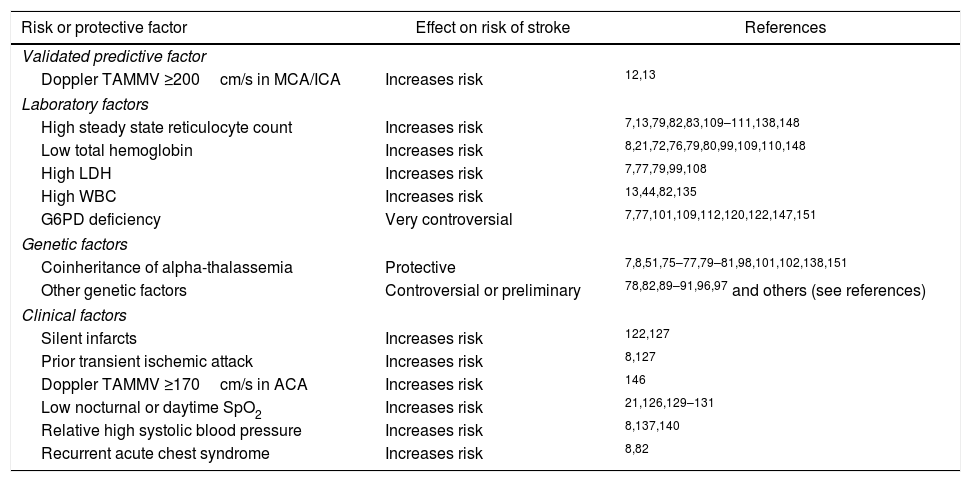
Main risk or protective factors for overt ischemic stroke in sickle cell disease.
| Risk or protective factor | Effect on risk of stroke | References |
|---|---|---|
| Validated predictive factor | ||
| Doppler TAMMV ≥200cm/s in MCA/ICA | Increases risk | 12,13 |
| Laboratory factors | ||
| High steady state reticulocyte count | Increases risk | 7,13,79,82,83,109–111,138,148 |
| Low total hemoglobin | Increases risk | 8,21,72,76,79,80,99,109,110,148 |
| High LDH | Increases risk | 7,77,79,99,108 |
| High WBC | Increases risk | 13,44,82,135 |
| G6PD deficiency | Very controversial | 7,77,101,109,112,120,122,147,151 |
| Genetic factors | ||
| Coinheritance of alpha-thalassemia | Protective | 7,8,51,75–77,79–81,98,101,102,138,151 |
| Other genetic factors | Controversial or preliminary | 78,82,89–91,96,97 and others (see references) |
| Clinical factors | ||
| Silent infarcts | Increases risk | 122,127 |
| Prior transient ischemic attack | Increases risk | 8,127 |
| Doppler TAMMV ≥170cm/s in ACA | Increases risk | 146 |
| Low nocturnal or daytime SpO2 | Increases risk | 21,126,129–131 |
| Relative high systolic blood pressure | Increases risk | 8,137,140 |
| Recurrent acute chest syndrome | Increases risk | 8,82 |
TAMMV: time averaged maximum mean velocity; MCA: middle cerebral artery; ICA: internal carotid artery; LDH: lactate dehydrogenase; WBC: white blood cell; G6PD: glucose-6-phosphate dehydrogenase; ACA: anterior cerebral artery; SpO2: peripheral capillary oxygen saturation.
The pathogenesis of stroke in SCD probably involves a combination of the βS mutation of the HBB gene, genetic modifiers, and environmental factors. Genetic predisposition to stroke in SCD has been suggested by the fact that the occurrence of stroke among siblings and twins is increased.71,72
Stroke risk in individuals with SCD probably involves many genes, within and outside of the HBB locus. Genes involved in the process of inflammation, immune response, coagulation, cell adhesion, lipid metabolism, blood pressure regulation, hypoxia, among others, are candidates involved in the development of stroke. These genes have been associated with increased susceptibility or protection against the occurrence of the event.73 Evidence has been shown that inflammation is the most important process in the arteries of the Willis circle related to the development of stroke.74
The protective effect of alpha-thalassemia against the development of stroke is well established in the literature.7,8,51,75–81 This association has been attributed to both hematologic and rheologic factors. Due to the lower production of alpha globin chains, the co-inheritance of alpha-thalassemia reduces the intracellular concentration of Hb and, consequently, reduces polymerization, modulating other hematological characteristics. Most studies show an increase in the number of RBCs, total Hb and hematocrit levels, and decreased levels of mean corpuscular volume, mean corpuscular Hb, mean corpuscular Hb concentration, reticulocytes, leukocytes and hemolysis. Currently, a high reticulocyte count is considered the most important risk factor for stroke, as analyzed later in this review. A high reticulocyte count was a risk factor for acute cerebral ischemia and high-risk TCD in a multivariate analysis.82,83 Thus, the reduction of reticulocyte count caused by the co-inheritance of alpha-thalassemia51 may be crucial to protect children with SCA from stroke.
The deformability of sickled cells in individuals who also have alpha-thalassemia is greater than that of patients without it.84,85 In addition, individuals with alpha-thalassemia have reduced numbers of dense cells and irreversibly sickled cells.86–88 Furthermore, the presence of alpha-thalassemia could reduce the adhesion of sickled cells to the endothelium in vivo.39,40 Thus, the improvement in rheological features of RBCs conferred by the co-inheritance of alpha-thalassemia may also contribute to reducing the risk of stroke in children with SCA.
In the last few years, some studies have suggested a role of other genes in the pathogenesis of stroke in children with SCA. In a candidate gene association study, Sebastiani et al. used Bayesian network modeling that tested 108 single nucleotide polymorphisms (SNPs) in 39 genes and found that 31 SNPs in 12 genes interact with Hb F to modulate the risk of stroke. The predictive value of the model was assessed in an independent validation set of patients with SCA and it predicted the occurrence of stroke with a 100% true positive rate and a 98.14% true negative rate giving an overall predictive accuracy of 98.2%. Polymorphisms in the ADCY9, ANXA2, BMP6, CCL2, CSF2, ECE1, ERG, MET, SELP, TEK and TGFBR3 genes were associated with stroke and had a major statistically independent effect on the risk of event. Furthermore, polymorphisms in genes previously associated with stroke in populations without SCA, such as transforming growth factor (TGF)-beta pathway genes, were also included in the network.89
In a genome-wide association and exome study using a discovery cohort of 677 children and an independent validation cohort of 288 children, two mutations in the GOLGB1 (Y1212C) and ENPP1 (K173Q) genes were significantly associated with a decreased risk for stroke. The GOLGB1 Y1212C mutation was also associated with protection from silent infarcts and abnormal TCD.90 In a recent longitudinal study by our group, the ENPP1 K173Q was associated with an increased risk of stroke and trends toward increased risk for high-risk TCD.91 The ENPP1 is a class II glycoprotein known to influence insulin sensitivity, binding, and, consequently, inhibiting the insulin receptor. The variant ENPP1 173Q is a more potent inhibitor of the insulin receptor than the wild variant, and it has been associated with insulin resistance and diabetes type II.66,92 Insulin stimulates the activation of protein kinase B and this protein signals the release of NO from endothelial cells. This pathway is impaired in individuals with the ENPP1 173Q variant. This variant has also been associated with increased blood pressure, cardiovascular events, and reduced activity of enzyme nitric oxide synthase.93,94 We have proposed that NO pathway impairment is the possible mechanism for the ENPP1 K173Q modulation of stroke in children with SCA.91 Further studies are needed to shed additional light on the role of ENPP1 K173Q in the pathogenesis of stroke in pediatric patients with SCA.
Although several studies have reported that other genetic markers are associated with risk of stroke in individuals with SCD (Table 1), there is controversy over the different studies. Most of these genetic associations are still preliminary and require confirmatory studies.2 Few studies have been validated, mainly because the interpretation of these studies is hampered by the relatively small sample size and/or absence of cohorts to validate the results.95 In addition, there is a wide variation in the definition of the outcomes studied. Furthermore, stroke in children with SCD seems to be a complex multifactorial and polygenic disorder that is influenced by many characteristics, each with only modest effects. The small effect of each marker also contributes to the controversial results. The influence of the tumor necrosis factor-alpha (TNF-α) G-308A (rs1800629) polymorphism is a good example of these controversies. Some reports have indicated that homozygous for the −308G allele is associated with increased risk of stroke.96,97 On the other hand, the −308A allele was reported to be associated with increased risk of stroke in two recent reports from our group82,98 whereas two other studies reported no association.99,100 Similarly, some studies have identified an association of the Central African Republic (CAR) haplotype with stroke in subjects with SCD.79,101–103 However, reliable replication of this potential association has not been reached in other independent validation cohorts.78,104–107
Regarding laboratory parameters, the relationship of hemolysis markers, such as low Hb concentration, high lactate dehydrogenase (LDH), and high reticulocyte count, with the occurrence of stroke stands out in the literature.7,8,13,19,76,77,79,80,83,99,108,109 Recently, the reticulocyte count has gained prominence as possibly the most important laboratory risk factor for increased risk of stroke.7,82,83,110,111 In recently published reports, including two by our group, reticulocyte count was the most important predictor of stroke or high-risk TCD.82,83,110–112 The re-analyzed data from the Cooperative Study of Sickle Cell Disease report, designed to assess the impact of very early detection of reticulocytosis, anemia, or leukocytosis on prediction of future major adverse events, showed that a high reticulocyte count was significantly associated with increased risk of stroke and death during childhood.111 More recently, data from a French cohort of children demonstrated a substantial independent association of high reticulocyte count [Hazard ratio: 1.82 per 50×109/L increase; 95% confidence interval (95% CI): 1.10–3.01] with development of cerebral macrovasculopathy.112 In a Brazilian cohort of 395 children with SCA, multivariate analysis showed that for each percent increase in reticulocyte count, the mean risk of acute cerebral ischemia (stroke or transient ischemic attack – TIA) or high-risk TCD increased by approximately 1.3% (95% CI: 1.13–1.47%) and 1.5% (95% CI: 1.27–1.69%), respectively.82 Reticulocytes probably have an essential role in the pathogenesis of cerebral vasculopathy in children with SCA. Hyperhemolysis, as indicated by high steady state reticulocyte count, releases Hb, free heme, arginase, and other molecules from RBCs, which generate reactive oxygen species, scavenge NO, and inhibit NO production, promoting endothelial damage, platelet activation and induction of inflammation in the vascular endothelium.113–116 Endothelial dysfunction and inflammation stimulate selective release of mediators, which further promote expression of adhesion molecules on the endothelial and blood cells,117 contributing to a series of pathophysiological events that culminate in vasculopathy involving large cerebral arteries. Additionally, data from the French cohort showed that the serum LDH level and reticulocyte count were significant independent factors associated with stroke in multivariate analyses,7 suggesting that hemolysis is not the single event associated with the pathophysiology of stroke but high steady state reticulocyte count per se is also probably involved. The tendency of reticulocytes to adhere to endothelial cells, resulting in endothelial activation and damage, might be the first stage of a series of pathophysiological events resulting in cerebral vasculopathy.36 The benefits of hydroxyurea therapy in the prevention of stroke14,118 could be partially attributed to its effect on decreasing reticulocyte adhesion to endothelial cells.119
Despite the relatively large number of studies, the role of concomitant glucose-6-phosphate dehydrogenase (G6PD) deficiency on risk of stroke remains controversial. In our experience, the prevalence of stroke or high-risk TCD was not significantly different in the groups with and without G6PD deficiency in a retrospective cohort study.120 This absence of association has been reported in other studies that used molecular analysis as the diagnostic method for G6PD deficiency.78,121 However, Thangarajh et al.,122 showed that the presence of the 376G (rs1050829) or 202A (rs1050828) allele was a significant and independent risk factor for intracranial MRA-arteriopathy in males with SCA. The 376G allele leads to a very mild reduction in G6PD activity, as demonstrated in one study.120 Children with the 376G allele (isoform A in males or AA in females) showed 85.2% of the enzymatic activity of G6PD when compared to individuals with the wild 376A allele (isoform B in males or BB in females). On the other hand, studies that reported an association between G6PD deficiency and abnormal TCD or intracranial stenosis used the measurement of G6PD activity as the method to define G6PD deficiency.7,77 One plausible explanation for these divergent results is that transcriptional and epigenetic factors that influence G6PD expression123 may be involved in the modulation of stroke in children with SCA. Thus, children without pathogenic missense mutations, but with downregulation of G6PD expression might have a higher risk for CVD. Similarly, children with pathogenic missense mutations and upregulation of G6PD expression might have a lower risk. However, some studies108,120,121,124 did not detect any difference in the mean G6PD activity in groups with and without ischemic stroke or high-risk TCD. The association of G6PD deficiency and stroke seems to be unlikely. The most important laboratory predictor of stroke, high steady state reticulocyte count,83,111 is associated with raised G6PD activity, as demonstrated in one study.120 Another reason for the improbable association between G6PD deficiency and stroke is that G6PD deficiency is an X-linked inherited disease and the effects of deficiency should be more common in males. However, available data show no influence of gender.108 It is obvious that genetic background heterogeneity among populations may also lead to contradictory results. G6PD deficiency may be a risk factor for some children, but not for others from different ethnic backgrounds. Further large-scale prospective longitudinal studies controlled for ancestry are warranted to elucidate the relationship of G6PD deficiency with stroke susceptibility in children with SCA. Furthermore, G6PD seems to have a critical antioxidant role in endothelial cells.7 Further studies measuring the G6PD activity in circulating endothelial cells may provide a better understanding of the relationship between G6PD deficiency and cerebrovascular vasculopathy in SCA.
About clinical features associated to the development of stroke, factors that cause imbalance between demand and supply of oxygen in the brain are critical. For example, aplastic crisis secondary to erythrovirus B19 infection,125 nocturnal hypoxemia,126 and the occurrence of acute chest syndrome8 have been reported to be associated with stroke. Additionally, in a large study of 516 children with SCA, silent cerebral infarct was the single factor associated with intracranial MRA-vasculopathy in the final multivariate logistic regression model.122 Another study of 248 children with SCA reported a strong association between silent infarcts identified at age of six years or older and subsequent development of stroke.127 Although both events share some pathophysiological background, the reason for this association is not clear. More convincingly, patients with a history of TIA were much more likely to have an overt stroke.8,127 According to data from the Cooperative Study of Sickle Cell Disease (CSSD), children who have had a TIA have a 56-times higher chance to subsequently develop stroke when compared to those without prior TIA (95% CI: 12.0–285).8 In our experience,82 four children had TIA and subsequently developed strokes and another three children had TIA and did not evolve with strokes; of the latter group the TIA was followed by a high-risk TCD (two cases) or an inconclusive TCD (one case) due to difficulty of insonation in the presence of a ‘good’ transtemporal window, probably due to severe stenosis.128 Then, recognizing and treating TIA certainly reduces the risk of an overt stroke. Children with a TIA event should be intensively monitored by TCD and/or MRA to evaluate the indication of intensification therapy such as prophylactic blood transfusion program or hydroxyurea therapy. Another clinical factor that has been associated with the occurrence of stroke is nocturnal126 or daytime129–131 Hb desaturation. Hypoxemia stimulates and exacerbates pathophysiological events involved in the vasculopathy associated with stroke in SCD, including Hb S polymerization, platelet activation, endothelial adhesion, and adhesion of RBCs to the endothelium.132–134
ConclusionsThe scientific literature is controversial in relation to the risk factors associated with the development of stroke in individuals with SCD. The absence of uniformity and standardization in the definition of distinct CVD events (ischemic stroke, TIA, hemorrhagic stroke, silent infarcts, vascular stenosis detected by MRA, and moya-moya disease) makes the interpretation and comparison of study results difficult and is probably a major factor that explains the controversies.
To date, the reticulocyte count in the peripheral blood is probably the most important laboratory marker to predict the occurrence of stroke in individuals with SCD. Clinical factors associated with hypoxia and reduced availability of oxygen in the brain play an important role in the pathophysiology of stroke and may act as a triggering factor for sudden acute cerebrovascular events. Although promising, genetic factors have a small effect on the occurrence of stroke when assessed individually. These factors may be used as a prognostic clinical tool in personalized medicine and may assist in the early detection of stroke risk in individuals with SCD, improving the selection of children for intensification therapy. For this purpose, they must be used all together, for example, as in the Bayesian network proposed by Sebastiani et al.89
Currently, the only validated prognostic clinical tool available for assessing the risk of stroke is TCD. Prospective validation studies must be conducted before including other biomarkers in the guidelines for clinical management of children with SCD.