Carica papaya Linn. has high nutraceutical and pharmacological values. The leaves possess antimicrobial, anti-tumor and antioxidant properties. They are used to treat thrombocytopenia during dengue fever and the leaf extract is commercially available as tablets under the name Caripill™ (MicroLabs, Bengaluru). Nevertheless, platelet transfusion is recommended in severe cases of thrombocytopenia, but the platelet storage is limited to 5–7 days at 22−24°C. Reducing oxidative stress (OS) during platelet storage might help in prolonging the shelf-life, since the OS is known to cause platelet storage lesion. Hence, this study investigated the effects of Caripill™ as an additive in Tyrode’s buffer during extended platelet storage.
MethodsPlatelets isolated from 4 months old male Wistar rats were stored with Caripill™ (50 and 100μg/ml) at 22°C for 12 days. Platelet functional and metabolic markers and various OS markers were analyzed on days 0, 4, 8 and 12.
ResultsCaripill™ (50 and 100μg/ml) maintained platelet functions and lactate dehydrogenase, elevated nitrites, reduced glucose consumption, protected proteins and up-regulated the antioxidant enzymes. However, the CP100 up-regulated catalase from day 4, elevated nitrites from day 8, prevented the formation of secondary products of lipid peroxidation and increased the total antioxidant capacity on day 4.
ConclusionsCaripill™ reduced platelet storage lesion up to day 8 of storage. Results suggest that a higher concentration of Caripill™ was more effective in combating the oxidative damage during platelet storage. This study throws light on the beneficial effects of Caripill™ in combating oxidative stress during platelet storage.
Carica papaya Linn (Family: Caricaceae) has high nutraceutical and pharmacological values. The leaves of C. papaya mainly contain phenolic acids, flavonoids, caffeic acid, protocatechuic acid, quercetin, kaempferol and 5,7-dimethoxycoumarin and possess antimicrobial, anti-tumor and antioxidant properties.
The C. papaya leaves are used for the treatment of thrombocytopenia during dengue fever.1 The C. papaya leaf extract, commercially available as Caripill™ (MicroLabs, Bengaluru), is also used during treatment of dengue fever. The papaya leaves possess membrane-stabilizing properties that prevent stress-induced plasma membrane destruction, which is attributed to the flavonoids and other phenolic compounds. Caripill™ is also responsible for the high expression of the platelet-activating factor receptor (PTAFR) gene, which plays an important role in platelet production.2
Platelet transfusion is recommended during severe cases of thrombocytopenia. Platelets are stored at 22−24°C, however the storage period is limited to 5–7 days, mainly due to bacterial growth, along with the development of platelet storage lesions (PSLs).3 Oxidative stress (OS) is one of the major causes of the development of PSL. Therefore, reducing OS might assist in prolonging the storage period. Singh et al.4 have also reported that the 9% hexane extract of C. papaya leaves controlled the PSL and microbial contamination during the 7-day storage.
However, there are no reports on the use of Caripill™ during platelet storage. Platelets are stored for a very short period and increasing their efficacy or shelf-life can contribute towards better blood banking. Furthermore, platelet storage in Tyrode’s buffer leads to better cell preservation and aids in controlling the storage environment. The Tyrode’s buffer (free of calcium and magnesium) has also been shown to reduce activation of stored platelets and is similar to plasma in maintaining the in vitro platelet functions. Therefore, this study investigated the effects of Caripill™ as an additive in Tyrode’s buffer during extended platelet storage.
Materials and methodsChemicalsThe Caripill™ (Micro Labs Limited, Bangalore, India) was purchased from a local pharmacy. The thiobarbituric acid (TBA), epinephrine, Griess reagent and bathocuproinedisulfonic acid disodium salt (BCS) were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). The cytochrome C (Cyt C), collagen and adenosine triphosphate (ATP) were purchased from SRL Chemicals. All other chemicals were of reagent grade and organic solvents of spectral grade.
Blood samplingAnimals (male Wistar rats; 4 months old) were obtained from the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)-registered animal breeding center (841/b/04/CPCSEA). Blood was collected according to Devi et al.5 Blood was carefully aspirated from the heart of the animal into collection tubes containing CPDA-1 (citrate phosphate dextrose adenine).
Isolation of plateletsBlood was centrifuged at 2000rpm for 15min at room temperature. The platelet-rich-plasma (PRP) was then centrifuged (4000rpm; 15min; 22°C) and the Tyrode’s buffer (pH 7.4) was used to resuspend the platelet pellet.6
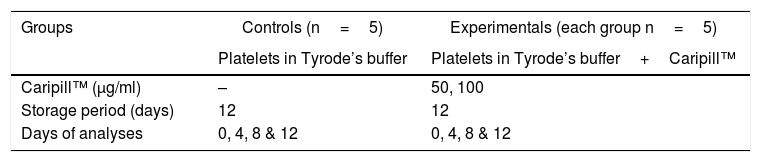
Experimental designIsolated platelets were divided into controls (n=5) and experimentals (n=5) and those of the experimentals were stored in the Tyrode’s buffer at 22−24°C with Caripill™ for a period of 12 days, as represented in Table 1.
Experimental design.
| Groups | Controls (n=5) | Experimentals (each group n=5) |
|---|---|---|
| Platelets in Tyrode’s buffer | Platelets in Tyrode’s buffer+Caripill™ | |
| Caripill™ (μg/ml) | – | 50, 100 |
| Storage period (days) | 12 | 12 |
| Days of analyses | 0, 4, 8 & 12 | 0, 4, 8 & 12 |
Tyrode’s buffer: NaCl: 140mM; KCl: 2.7mM; NaH2PO4: 0.4mM; NaHCO3: 11.9mM; Glucose: 11.1mM.
The platelets were incubated (with and without collagen (2.0μg/ml)) at 37°C, and the absorbance was measured at 405nm.7 The platelet aggregation was inversely proportional to the absorbance recorded. The decrease in absorbance indicated an increase in platelet aggregation and vice-versa.
ATP secretionThe platelets were incubated (37°C) with collagen (2.0μg/ml) and treated with perchloric acid (1.2M). The absorbance was read at 260nm and the amount of adenine nucleotides was calculated, using an ATP standard.8
Platelet quality and metabolismLactate dehydrogenase (LDH, EC 1.1.1.27)The platelets were treated with the mixture of Reagent 1 (tris, NaOH and pyruvate) and Reagent 2 (nicotinamide adenine dinucleotide (NAD)) and incubated at 37°C. The absorbance was measured at 340nm.9
pHThe pH in the stored platelets was determined by the Fisher Scientific pH strips.10
GlucoseThe glucose in the platelets was measured by glucose oxidase-peroxidase (GOD-POD) method by following the protocol described in the Autospan Gold kit and the absorbance was measured at 546nm.11
OS markersSuperoxidesThe platelets were incubated with 200μl cytochrome C (Cyt C) (160μM) at 37°C. The Cyt C reduction was measured spectrophotometrically at 550nm.12
NitritesThe platelets were treated with the Griess reagent and incubated in the dark at room temperature. The absorbance was measured at 548nm. Sodium nitrite was used as the standard to determine the amounts of nitrites.13
Lipid peroxidationConjugate dienesThe platelets were treated with ether/ethanol (1:3 (v/v)). The mixture was centrifuged and the absorbance was measured spectrophotometrically at 235nm.12
Thiobarbituric acid reactive substances (TBARS)The platelets, treated with 20% cold trichloro acetic acid (TCA) in 0.6M HCl, were centrifuged and 0.12M thiobarbituric acid (TBA) was added to the supernatant. The samples were incubated in a boiling water bath and the absorbance was read at 532nm.14
Protein oxidationProtein carbonyls (PrC)The protein carbonyls were measured according to Reznick and Packer15 and the absorbance was read at 370nm. The carbonyl content was calculated using the absorption coefficient 22,000M−1cm−1.
Protein sulfhydryls (P-SH)The protein sulfhydryls were measured, as described by Habeeb.16 Absorbance was read at 412nm and the amount of sulfhydryls was calculated using the molar absorptivity of 13,600M−1cm−1.
Antioxidant defensesSuperoxide dismutase (SOD, EC 1.15.1.1)The carbonate buffer (0.05M; pH 10.2; 0.1Mm ethylenediaminetetraacetic acid (EDTA)) was added to the platelets, followed by epinephrine, and the absorbance was measured at 480nm. The SOD activity was expressed as the amount of enzyme that inhibits oxidation of epinephrine by 50%, which is equal to 1 unit.17
Catalase (CAT, EC 1.11.1.6)The platelets were treated with absolute ethanol and incubated in an ice bath (30min). The phosphate buffer and H2O2 (0.066M) were added to the incubated samples and the absorbance was measured at 240nm. The catalase activity was determined using the molar extinction coefficient 43.6Mcm−1.18
Total antioxidant capacity (TAC) — cupric ion reducing antioxidant capacity (CUPRAC)The samples were treated with bathocuproinedisulfonic acid disodium salt (BCS) (0.25mM) and absorbance was measured at 490nm. The CuSO4 solution (0.5mM) and EDTA solution (0.01M) were added. Absorbance was read at 490nm and uric acid was used as the standard.19
Protein estimationThe platelet protein concentration was determined according to Lowry et al.20
Statistical analysesThe results are represented as Mean±SE (n=5). The two-way Analysis of Variance (ANOVA) was performed between the groups (storage days) and sub-groups (between concentrations). The results were considered significant at p<0.05. The Bonferroni Post Test was performed using the GraphPad Prism 6 Software (GraphPad Software Inc., California, USA).
Results- •
The first paragraph in the results of all the parameters is comprised of the significant changes between the groups during storage against day 0.
- •
The second paragraph is comprised of the significant changes within the group, i.e., between the concentrations (sub-groups) against their respective controls.
The interaction of platelets with agonists, such as collagen, is responsible for the physiological responses in platelets, such as aggregation, adhesion and secretion. These responses in stored platelets are some of the important aspects that determine the transfusion efficacy.
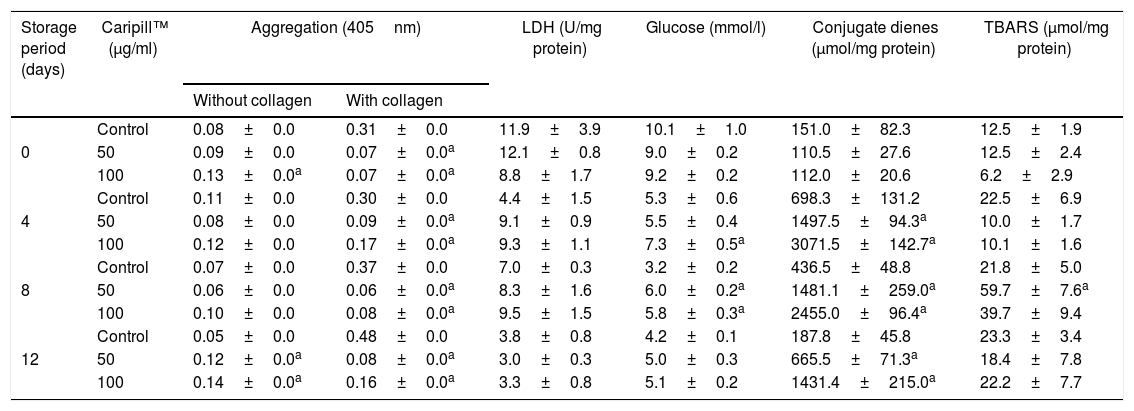
Aggregation (without collagen)The platelet aggregation (without collagen) was maintained in the control CP50 (Caripill™ 50μg/ml) and CP100 (Caripill™ 100μg/ml) throughout storage.
The aggregation declined by 70% on day 0 in CP100, as compared to the control. The aggregation showed a decrease of one-fold on day 12 in CP50 and CP100, compared to the control (p< 0.005) (Table 2).
Aggregation, lactate dehydrogenase, glucose and lipid peroxidation in platelets stored with Caripill™.
| Storage period (days) | Caripill™ (μg/ml) | Aggregation (405nm) | LDH (U/mg protein) | Glucose (mmol/l) | Conjugate dienes (μmol/mg protein) | TBARS (μmol/mg protein) | |
|---|---|---|---|---|---|---|---|
| Without collagen | With collagen | ||||||
| 0 | Control | 0.08±0.0 | 0.31±0.0 | 11.9±3.9 | 10.1±1.0 | 151.0±82.3 | 12.5±1.9 |
| 50 | 0.09±0.0 | 0.07±0.0a | 12.1±0.8 | 9.0±0.2 | 110.5±27.6 | 12.5±2.4 | |
| 100 | 0.13±0.0a | 0.07±0.0a | 8.8±1.7 | 9.2±0.2 | 112.0±20.6 | 6.2±2.9 | |
| 4 | Control | 0.11±0.0 | 0.30±0.0 | 4.4±1.5 | 5.3±0.6 | 698.3±131.2 | 22.5±6.9 |
| 50 | 0.08±0.0 | 0.09±0.0a | 9.1±0.9 | 5.5±0.4 | 1497.5±94.3a | 10.0±1.7 | |
| 100 | 0.12±0.0 | 0.17±0.0a | 9.3±1.1 | 7.3±0.5a | 3071.5±142.7a | 10.1±1.6 | |
| 8 | Control | 0.07±0.0 | 0.37±0.0 | 7.0±0.3 | 3.2±0.2 | 436.5±48.8 | 21.8±5.0 |
| 50 | 0.06±0.0 | 0.06±0.0a | 8.3±1.6 | 6.0±0.2a | 1481.1±259.0a | 59.7±7.6a | |
| 100 | 0.10±0.0 | 0.08±0.0a | 9.5±1.5 | 5.8±0.3a | 2455.0±96.4a | 39.7±9.4 | |
| 12 | Control | 0.05±0.0 | 0.48±0.0 | 3.8±0.8 | 4.2±0.1 | 187.8±45.8 | 23.3±3.4 |
| 50 | 0.12±0.0a | 0.08±0.0a | 3.0±0.3 | 5.0±0.3 | 665.5±71.3a | 18.4±7.8 | |
| 100 | 0.14±0.0a | 0.16±0.0a | 3.3±0.8 | 5.1±0.2 | 1431.4±215.0a | 22.2±7.7 | |
LDH: lactate dehydrogenase; TBARS: thiobarbituric acid reactive substances.
Changes between the groups (storage days) were significant in aggregation with collagen, LDH, glucose, conjugate dienes and TBARS (p<0.05).
The platelet aggregation (with collagen) declined in the control by 20% and 50% on days 8 and 12, respectively, and in CP100 by 20% and 10% on days 4 and 12, respectively, compared to day 0.
The platelets aggregated by 75% in CP50 and CP100 on day 0, with respect to the control. The aggregation also increased by 70% and 40% in CP50 and CP100, respectively, on day 4, compared to the control. Increments of ∼80% was observed on days 8 and 12 in CP50 and CP100, respectively, with corresponding controls (p<0.0001) (Table 2).
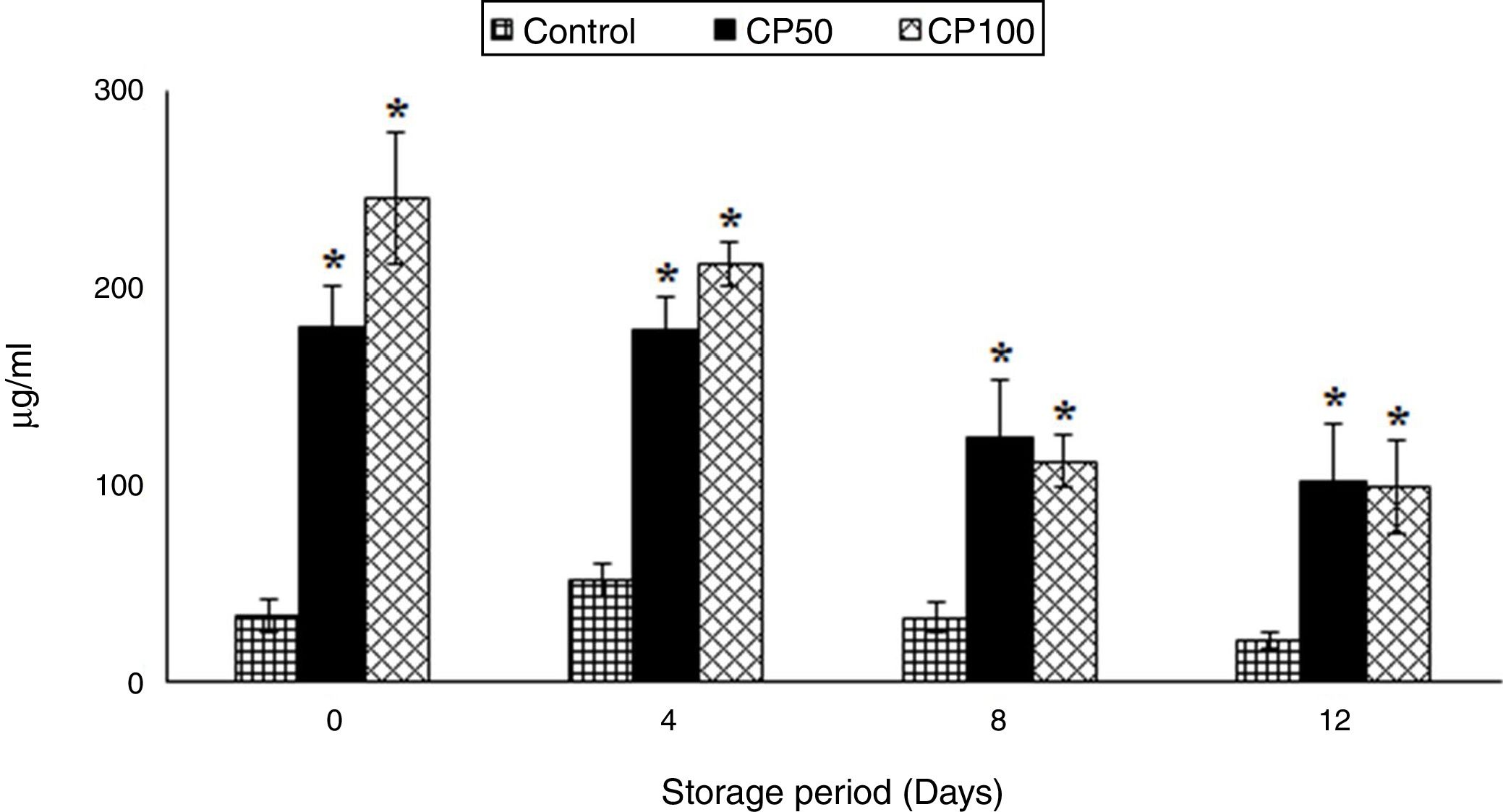
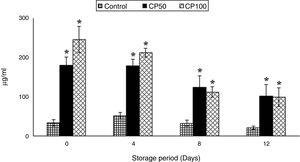
ATP secretionThe ATP secretion in CP100 decreased by 60% on days 8 and 12, when compared to day 0.
The ATP secretion increased 4-fold in CP50 (Caripill™ 50μg/ml) and 6-fold in CP100 (Caripill™ 100μg/ml) on day 0, compared to the control. Elevations of 3-fold and 2-fold were also observed on days 4 and 8, respectively, in CP50 and CP100, compared to their controls (p<0.0001) (Figure 1).
ATP secretion in platelets stored with Caripill™.
CP50: Caripill™ 50μg/ml; CP100: Caripill™ 100μg/ml.
Changes between the groups (storage days) were significant in ATP secretion. (p<0.0001).
*Represents significant changes within the group (i.e., between antioxidant concentrations), when compared with respective controls.
Elevations in the glucose consumption during platelet storage leads to decreased pH and ATP levels, leading to accelerated platelet lysis. Changes in pH are shown to affect platelet aggregation response. The LDH is released during platelet activation under OS in conditions such as storage, eventually causing morphological changes in platelets.
Lactate dehydrogenase (LDH)The LDH activity declined by 65% in the control on days 4 and 12, compared to day 0. The CP50 and CP100 showed decrements of 75% and 60%, respectively, on day 12, compared to day 0 (Table 2).
pHThe control and Caripill™ groups showed elevations in pH during storage (p<0.0001). The pH increased by 30% (control) and 20% (CP50 and CP100) on days 8 and 12, compared to day 0 (∼6.85). However, it was maintained at 7.4 in CP50 up to day 4.
An elevation of 15% was observed on day 8 in the CP50 and CP100, with respect to the control.
GlucoseThe glucose decreased significantly (p<0.0001) in all groups during storage. The controls showed decrements of 50%, 60% and 70% on days 4, 8 and 12, respectively, compared to day 0. The glucose also decreased by 40% on days 4, 8 and 12 in the CP50, whereas in the CP100, this occurred on days 8 and 12, compared to day 0.
The CP100 showed an increase of 40% on day 4 and 85% on day 8 in all the Caripill™ groups, compared to their respective controls (Table 2).
OS markersFree radicalsThe superoxides play a major role in oxidation of low-density lipoproteins, which can lead to the activation of stored platelets. The nitric oxide (NO) is a potent signaling molecule that can alter platelet functions. Therefore, it is necessary to study the changes in NO or its metabolites, such as nitrites, during platelet storage.
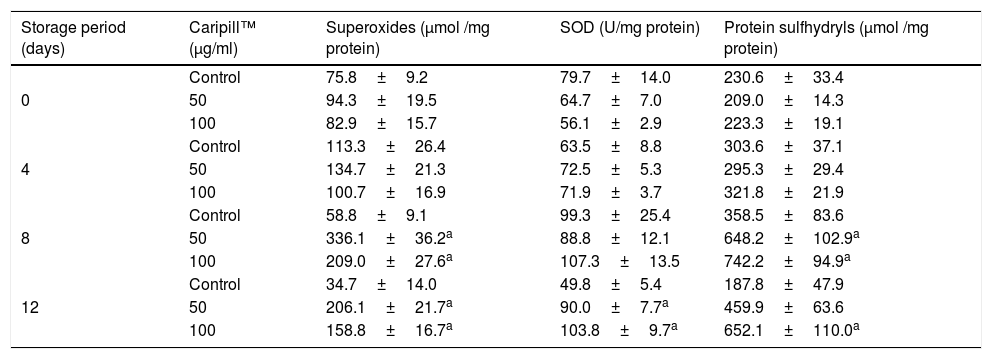
SuperoxidesThe CP50 showed a 2-fold increment on days 8 and 12 (end of the storage period), while a one-fold increase was observed in the CP100 on day 8, compared to day 0.
The superoxides also increased by 4- and 3-fold in the CP50 (Caripill™ 50μg/ml) and CP100 (Caripill™ 100μg/ml), respectively, on days 8 and 12, compared to the corresponding controls (p<0.0001) (Table 3).
Superoxides, superoxide dismutase and protein sulfhydryls in platelets stored with Caripill™.
| Storage period (days) | Caripill™ (μg/ml) | Superoxides (μmol /mg protein) | SOD (U/mg protein) | Protein sulfhydryls (μmol /mg protein) |
|---|---|---|---|---|
| 0 | Control | 75.8±9.2 | 79.7±14.0 | 230.6±33.4 |
| 50 | 94.3±19.5 | 64.7±7.0 | 209.0±14.3 | |
| 100 | 82.9±15.7 | 56.1±2.9 | 223.3±19.1 | |
| 4 | Control | 113.3±26.4 | 63.5±8.8 | 303.6±37.1 |
| 50 | 134.7±21.3 | 72.5±5.3 | 295.3±29.4 | |
| 100 | 100.7±16.9 | 71.9±3.7 | 321.8±21.9 | |
| 8 | Control | 58.8±9.1 | 99.3±25.4 | 358.5±83.6 |
| 50 | 336.1±36.2a | 88.8±12.1 | 648.2±102.9a | |
| 100 | 209.0±27.6a | 107.3±13.5 | 742.2±94.9a | |
| 12 | Control | 34.7±14.0 | 49.8±5.4 | 187.8±47.9 |
| 50 | 206.1±21.7a | 90.0±7.7a | 459.9±63.6 | |
| 100 | 158.8±16.7a | 103.8±9.7a | 652.1±110.0a |
SOD: superoxide dismutase.
Changes between the groups (storage days) were significant in superoxides, SOD, and protein sulfhydryls (p<0.05).
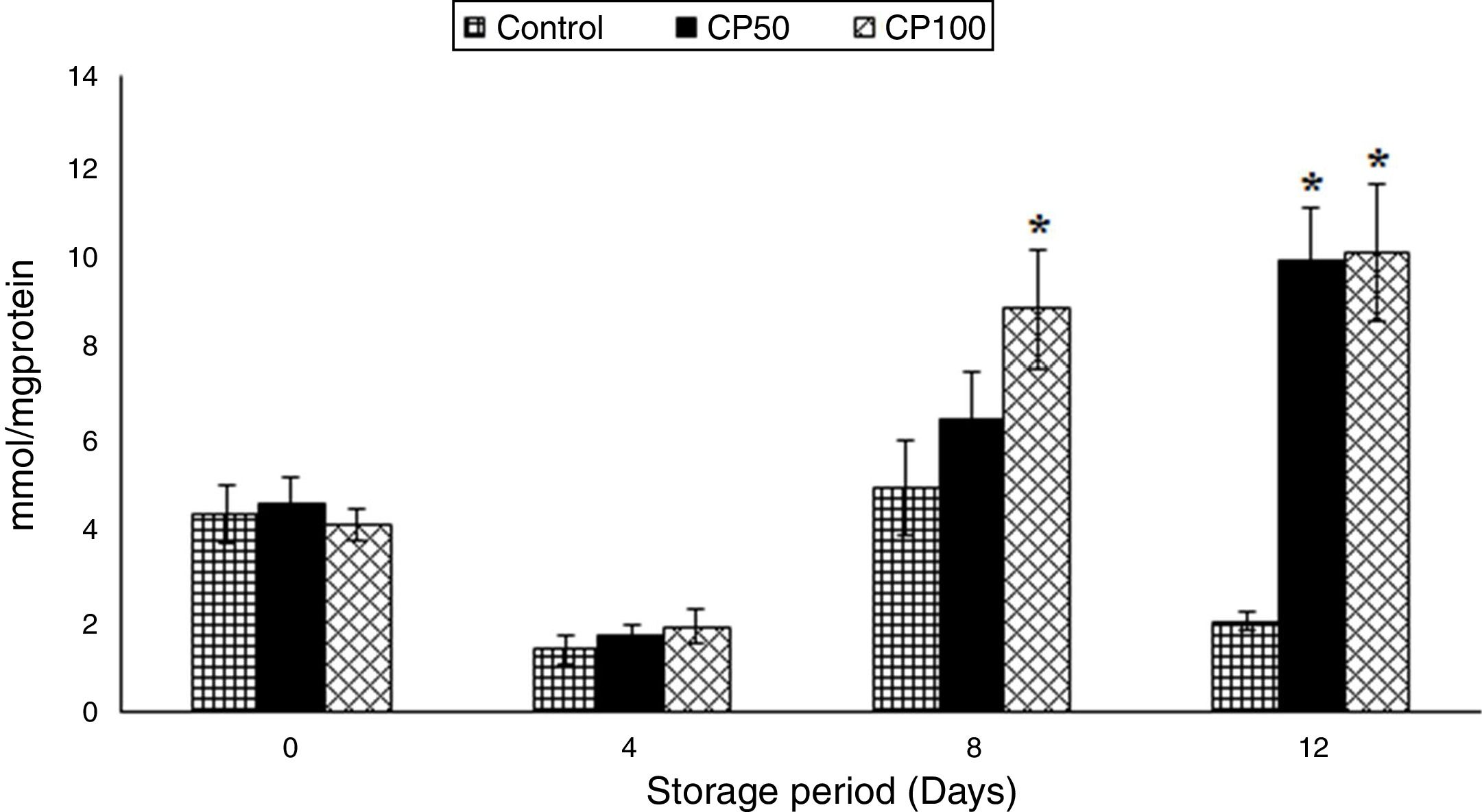
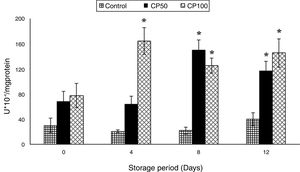
The nitrites increased in the Caripill™ groups by one-fold on days 8 and 12 (p=0.0001).
The nitrites increased by 80% in the CP100 on day 8 and 4-fold in the CP50 and CP100 on day 12 (p<0.0001), compared to their respective controls (Figure 2).
Nitrites in platelets stored with Caripill™.
CP50: Caripill™ 50μg/ml; CP100: Caripill™ 100μg/ml.
Changes between the groups (storage days) were significant in nitrites. (p<0.0001).
*Represents significant changes within the group (i.e., between antioxidant concentrations), when compared with respective controls.
The conjugate dienes and TBARS are the primary and secondary products of lipid peroxidation that act as the indices of oxidative damage. Therefore, their estimation aids in understanding the extent of the PSLs.
Conjugate dienesThe conjugate dienes increased by 12-fold (days 4 and 8) and 5-fold (day 12) in the CP50 and by 25-fold (days 4 and 8) and 10-fold (day 12) in the CP100, compared to day 0 (p<0.0001).
The CP50 and CP100 showed increments of 1- and 3-fold, respectively, on day 4, compared to the control. The conjugate dienes also increased by 2-fold in the CP50 and ∼5-fold in the CP100 on days 8 and 12, respectively, compared to their controls (p<0.0001) (Table 2).
Thiobarbituric acid reactive substances (TBARS)The variations were similar in all the groups on days 0, 4 and 12; however, the CP50 and CP100 showed significant elevations of 3- and 5-fold, respectively, on day 8, compared to day 0 (p<0.0001).
The TBARS increased by one-fold in the CP50 (Caripill™ 50μg/ml), compared to the control and decreased by 35% in the CP100 (Caripill™ 100μg/ml), compared to the CP50 on day 8 (Table 2).
Protein oxidationThe reactive oxygen species (ROS) regulate platelet activation and modulate platelet functions by inducing oxidation of thiols and carboxylation of platelet proteins. Estimation of protein carbonyls and sulfhydryls serve as markers to analyze the oxidative damage to proteins during storage.
Protein carbonyls (PrC)The carbonyls were 23.7μmol/mg protein in the control, 26.5μmol/mg protein in the CP50 and 29.0μmol/mg protein in the CP100 on day 0 and similar values were observed throughout storage in all the groups.
Protein sulfhydryls (P-SH)The protein sulfhydryls showed a decrement of 30% in the control on day 12, compared to day 0. An increment of ∼95% was observed in the CP50 on days 4, 8 and 12, compared to day 0. The CP100 showed increases of 45% and 20% on days 4 and 12, respectively, compared to day 0 (p<0.0001).
The sulfhydryls decremented by 50% in the CP50 on day 0, compared to the control. The CP50 and CP100 showed an elevation of ∼40% on day 12, compared to the control (Table 3).
Antioxidant statusThe antioxidant enzymes SOD and CAT form the first line of defense against oxidative damage and are significant markers of the antioxidant status. The platelets also possess non-enzymatic antioxidants, which include glutathione, vitamin C, vitamin E and other polyphenols.
Superoxide dismutase (SOD)The SOD activity in the CP100 increased by 90% at the end of storage.
The CP50 and CP100 showed increments of 80% and 100%, respectively, on day 12, compared to the control (Table 3).
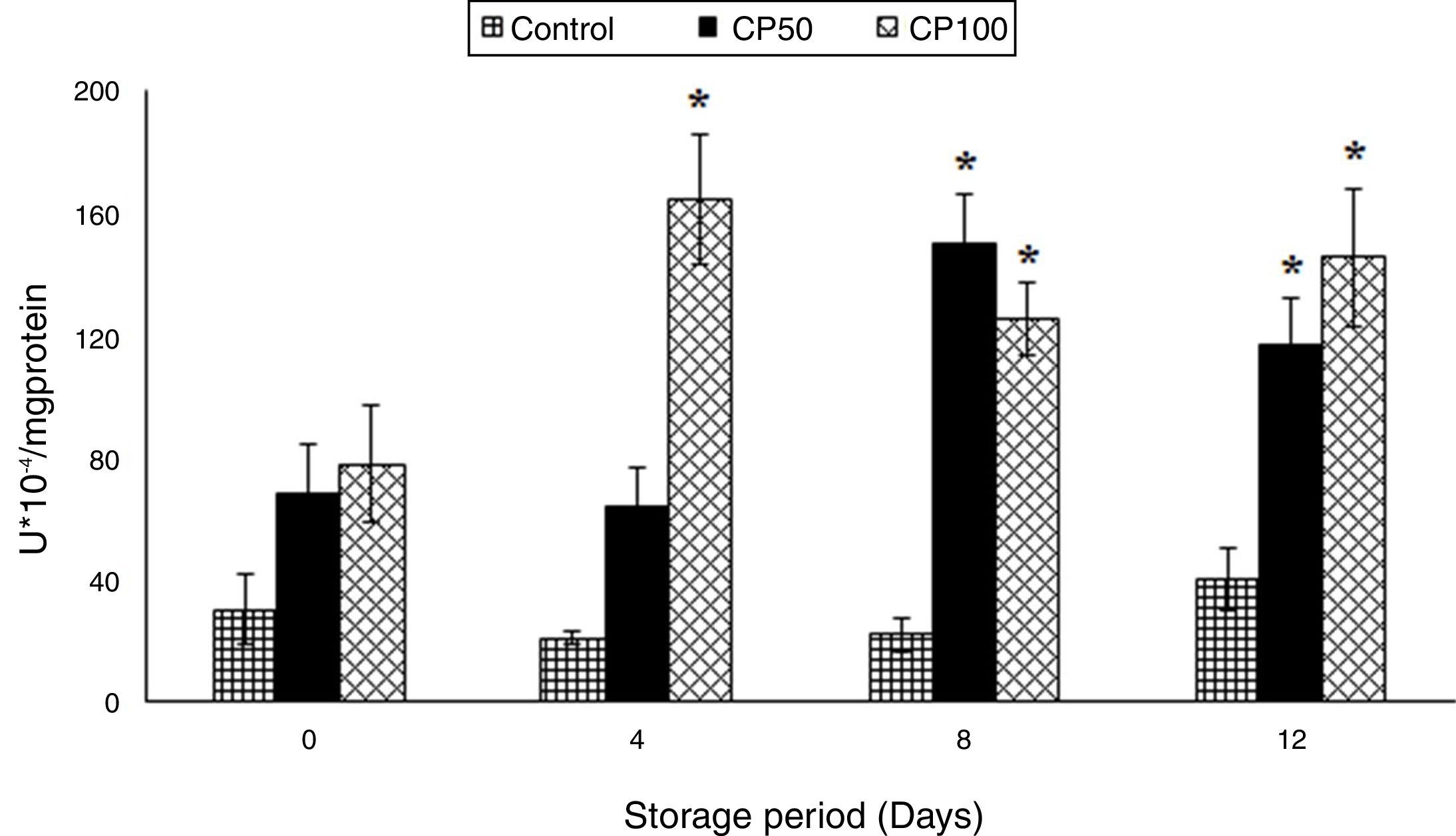
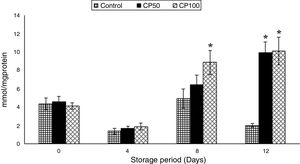
Catalase (CAT)The CAT increased by 95% on day 8 in the CP50 and on days 4 and 12 in the CP100, compared to day 0.
The catalase increased by 6-fold on day 4 in the CP100, compared to the control. The CP50 and CP100 showed increments of 5- and 4-fold, respectively, on day 8 and ∼2-fold on day 12, compared to the corresponding controls (p<0.0001) (Figure 3).
Catalase in platelets stored with Caripill™.
CP50: Caripill™ 50μg/ml; CP100: Caripill™ 100μg/ml.
Changes between the groups (storage days) were significant in catalase. (p<0.0001).
*Represents significant changes within the group (i.e. between antioxidant concentrations), when compared with respective controls.
The TAC increased in the CP100 from 230.3μmol uric acid equivalents/l on day 0–537.8μmol uric acid equivalents/l on day 4.
The CP50 and CP100 showed increments of 2- and 3-fold, respectively, on day 4, compared to the control (p=0.0002).
DiscussionCaripill™ is made from the C. papaya leaf extract, which has numerous medicinal properties and is used to treat thrombocytopenia during dengue and malaria. This study mainly focused on the effects of Caripill™ on the oxidative stress of stored platelets, along with platelet functional and metabolic markers.
The aggregation (without collagen) declined in platelets stored with Caripill™, when compared with the control, as the membrane stabilizing property of C. papaya2 prevents platelets from activating and aggregating. Decrements in platelet aggregation in the CP100 on day 0 further demonstrated that a higher concentration of Caripill™ can reduce spontaneous platelet aggregation from the beginning of the storage period. The results obtained are in accordance with the results of Chinnappan et al.,21 who also reported a decrease in the platelet aggregation when plasma was treated with the C. papaya leaf extract. However, when aggregation was induced using collagen, platelets stored with Caripill™ aggregated better than the controls even during an extended storage period. Thus, Caripill™ was beneficial in maintaining the platelet aggregation with an agonist during extended storage.
The ATP secretion increased in platelets stored with Caripill™, when compared with the control during storage. The magnesium present in C. papaya leaves is known to influence mitochondrial ATP synthesis.22 The ATP secretion in response to agonists, such as collagen, is one of the important platelet functions and Caripill™ can elevate the ATP secretion during storage.
The decrements in glucose during storage are due to the increased glucose consumption by platelets.23 The increase in the glucose levels in the Caripill™ groups on day 4 (CP100) and day 8 (CP50 & CP100), when compared with their respective controls, could have been due to reduced glucose consumption. Therefore, the increase in glucose demonstrates the ability of the C. papaya leaf extract to reduce the glycolytic activity during the platelet storage. The LDH, which decreased in all groups on day 12, is an indicator of membrane damage. Furthermore, a direct relation was observed between the LDH and glucose (r=0.810). The ability of Caripill™ to reduce the LDH can be one of the contributing factors to its membrane stabilizing property. The decrement in LDH in the control on day 4 could have been due to the activity of endogenous antioxidants, as ROS increase from day 8 of storage (from our previous storage study). The reduction in anaerobic glycolysis can extend the storage period of platelets.24
The changes in the pH of stored platelets can transform their shape and reduce the in vivo recovery after transfusion. The pH rose in all the groups during storage, although it was maintained at 7.4 in the CP50 only up to day 4. The platelets with a pH below 6.0 is not recommended by the Drug and Cosmetics Act of India24 and the pH of platelets stored with Caripill™ is in accordance with this standard.
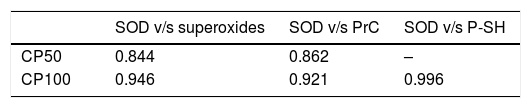
Elevations in the SOD activity on day 12 is in accordance with the elevated superoxides on day 8, which was further confirmed by the correlation between the SOD and superoxides (Table 4). The CAT detoxifies the H2O2 by degrading it into H2O and O2. The H2O2 can be produced by the dismutation of superoxides (both enzymatic and non-enzymatic), monoamine oxidases and β-oxidation of fatty acids by the peroxisomal pathway.25 The rise in CAT activity suggests the presence of high amounts of H2O2, which can be attributed to the dismutation of superoxides to release H2O2. Papaya leaves also contain saponins26 that can activate the antioxidant enzymes SOD and CAT by donating hydrogen ions.
Nitrites are the products of oxidative metabolism of NO.27 Our results showed a direct correlation between nitrites and superoxides (r=0.815), suggesting that the increments in nitrites from day 8 is a result of the breakdown of NO into nitrites due to the increase in ROS.
Papaya leaves are known to prevent lipid peroxidation.28 However, elevations in the conjugate dienes and TBARS indicate that the oxidative damage overwhelmed the antioxidant capacity of Caripill™. The TBARS decreased in the CP100 on day 8, indicating that higher concentration of Caripill™ could prevent the formation of secondary lipid peroxidation products. Sulfhydryls are the potential sites of reversible oxidative modification by S-thiolation and S-nitrosylation. The decrease in the sulfhydryls in the CP50 on day 0 can be due to the variations in the oxidative stress in the individual samples. However, the sulfhydryls increased in the CP50 and CP100 throughout the storage, while it decreased in the control on day 12, indicating that Caripill™ can successfully scavenge the free radicals, resulting in the reduction of disulfide groups to sulfhydryls.
Furthermore, the reactive carbonyl groups in proteins are formed due to the oxidation of arginine, lysine, threonine, or proline by the ROS in platelets. The protein carbonyls were maintained in the CP50 and CP100 throughout storage, suggesting that Caripill™ can protect susceptible proteins from oxidative damage. Additionally, both the markers of protein oxidation showed a direct relationship with the SOD (Table 4). This can be attributed to the compounds, such as polyphenols and flavonoids, present in papaya leaves, that possess the antioxidant property.29 The structural arrangement of the hydroxyl groups contribute to the antioxidant potential of the phenolic compounds.30
The total antioxidant capacity was determined by the reduction of cupric ions to cuprous ions by the antioxidants present in the sample. The TAC elevations in Caripill™ (50 & 100μg/ml) on day 4 can be attributed to the vitamins B12, A & C, folic acid, saponins, tannins, glycosides, alkaloids, phenols and flavonoids present in the leaves of the C. papaya.28 The decrement in the TAC (days 8 and 12) can be due to elevations in the ROS.
ConclusionsCaripill™ can reduce the PSL in terms of lower glycolytic activity, protection of proteins from oxidative damage, up-regulation of the antioxidant potential and maintenance of platelet functions up to day 8 of storage. The CP100 was better in protecting lipids and upregulating the antioxidant defenses, suggesting that higher concentration of Caripill™ were more effective in combating the oxidative damage during the platelet storage. This study throws light on the beneficial effects of Caripill™ in combating oxidative stress during the platelet storage. However, its effects on bacterial growth and other platelet functions needs to be investigated.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge Dr. Leela Iyengar, Ms. Soumya Ravikumar, Mr. Carl Hsieh and JAIN (Deemed-to-be University) for their support.