The evolving COVID-19 pandemic became a hallmark in human history, not only by changing lifestyles, but also by enriching scientific knowledge on viral infection and its consequences.
ObjectiveAlthough the management of cardiorespiratory changes is pivotal to a favorable prognosis during severe clinical findings, dysregulation of other systems caused by SARS-CoV-2 infection may imbalance erythrocyte dynamics, such as a bidirectional positive feedback loop pathophysiology.
Method and ResultsRecent evidence shows that SARS-CoV-2 is capable of affecting the genetics and dynamics of erythrocytes and this coexists with a non-homeostatic function of cardiovascular, respiratory and renal systems during COVID-19. In hypothesis, SARS-CoV-2-induced systematical alterations of erythrocytes dynamics would constitute a setpoint for COVID-19-related multiple organ failure syndrome and death.
ConclusionThe present review covers the most frequent erythrocyte-related non-homeostatic findings during COVID-19 capable of providing mechanistic clues of SARS-CoV-2-induced infection and inspiring therapeutic-oriented scientific evidence.
In 2020, local disease control and prevention centers in Beijing unraveled an unknown pathogen: a new variation of coronavirus now known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The COVID-19 (Coronavirus Disease 19) pandemic has dramatically changed the way global health is being managed. Even with the wishful vaccines in the spotlight, worldwide data has shown that the COVID-19 pandemic is still uncontrolled.2,3 Hence, additional clinical and scientific efforts remain pertinent towards a better understanding of the pathophysiology of this disease. The persisting post-infection consequences on different systems further require more extensive knowledge on these pathophysiological processes.
The combination of diagnostic tools, composed of RT-PCR, serology, imaging tests and clinical evaluation may reveal the pathophysiology and clinical-epidemiological information to better guide clinical approaches and prognosis. Findings from these methods could provide important clues to the COVID-19 signature towards addressing many clinical and research questions, thus diminishing the life-threatening potential of the SARS-CoV-2 infection.4 A worth possibility that would aid to these clues may arise from concomitant hematological assessments.
The effects caused by the SARS-CoV-2 infection presents a complex combination of clinical manifestations, from mild flu-like symptoms to multiple organ failure and death.5 These multisystemic illnesses necessitate a complex set of approaches. According to Danying Liao and colleagues, clinical and laboratory findings suggest that refractory hypoxemia in critically ill patients can induce vasoconstriction prior to a reduction in blood flow and vascular occlusion. Altogether, this may reduce the tissue perfusion leading to notable clinical manifestations.6 Therefore, the search for potentially useful clues for the disease progression and prognosis management merit in-depth testing in blood samples. Although the clinical developments related to the SARS-CoV-2 infection keep growing, the COVID-19 pathophysiology is yet poorly correlated with the aggravating hematological changes, which may be the cause for many perfusion-dependent manifestations. The present work compiles the main pathophysiological mechanisms arising from the SARS-CoV-2 infection and relates them to the frequent hematological findings in COVID-19 patients. The working hypotheses in this review are aimed at inspiring the scientific community in the search for additional evidence on erythrocyte-related non-homeostatic control during the COVID-19 pandemic.
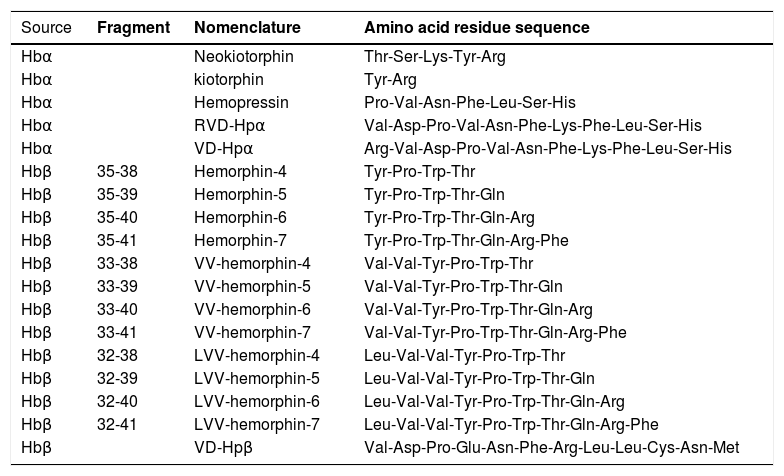
SARS-COV-2 infection from molecular StandpointSARS-CoV-2, an RNA +, is known to be responsible for respiratory infections5,7 and present spherical and enveloped morphology, whose structure is composed of: i) the peak glycoprotein S or Spike (S) protein, which covers the surface of the Sars-CoV-2 and serves as a tool to enter human cells (found in all CoVs); ii) hemagglutinin esterase (HE), expressed in some CoVs, including SASR-CoV-2; iii) the membrane protein (M), a type III transmembrane glycoprotein, and; iv) the envelope protein (E), located between the S proteins on the viral envelope7,8 (Figure 1).
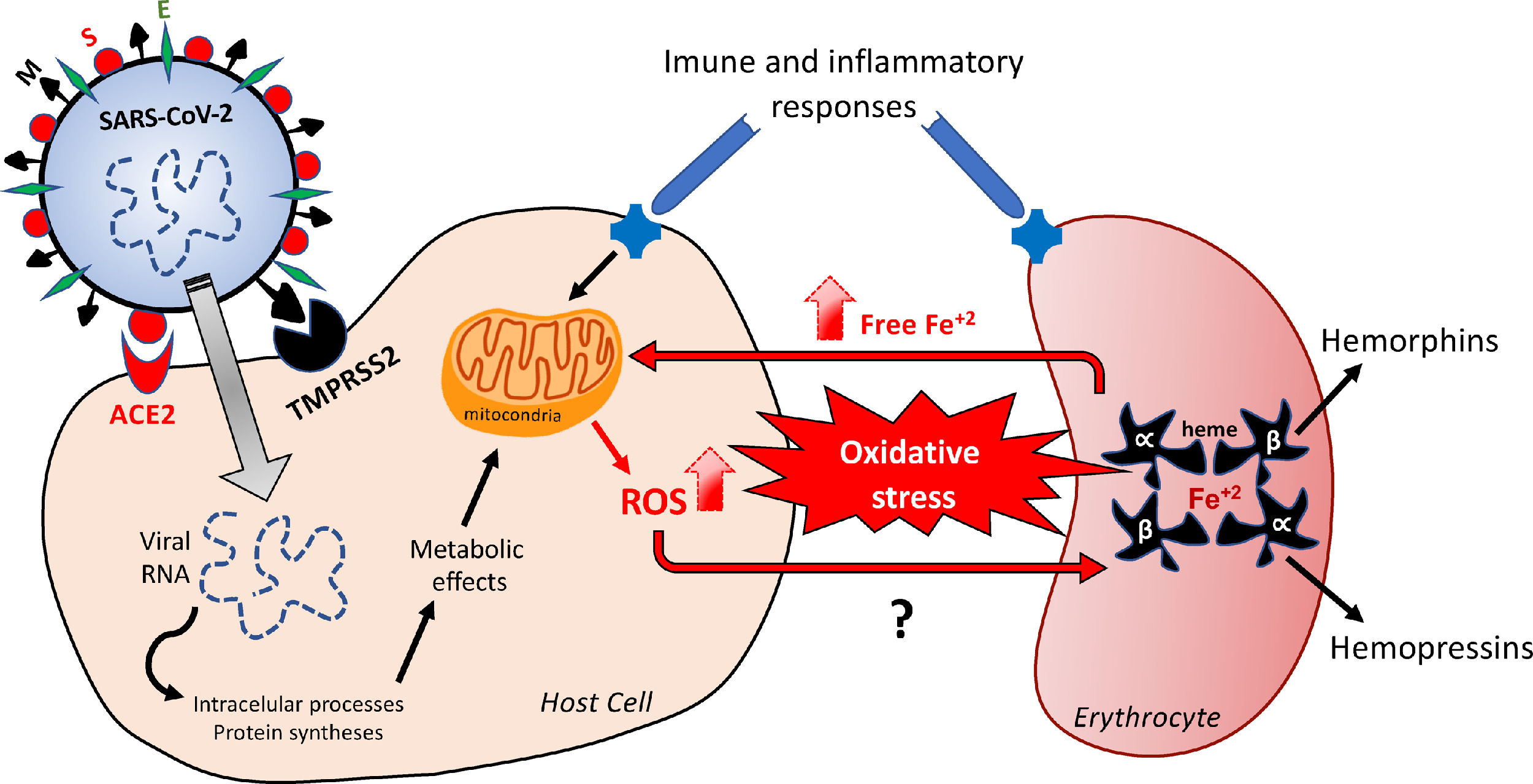
Hypothetical feedback loop oxidative mechanism resulting in, and from, erythrocyte death. The SARS-CoV-2 infection would activate metabolic processes inside the host cell and in inflammatory/immune systems that would result in an imbalanced redox status. Oxidative stress affects erythrocytes, thus inducing death of these red blood cells. The release of free iron, as a direct consequence of this erythrocyte destruction, would facilitate the metabolic reactions underlying the oxidative stress. The major structural proteins of SARS-CoV-2 interacting with host cells are spike (S), membrane (M) and envelope (E). Angiotensin-converting enzyme type 2 (ACE-2) and serine protease 2 (TMPRSS2) are the host cell membrane proteins interacting with SARS-CoV-2 proteins. Alpha and Beta are the hemoglobin chains released following erythrocyte death, from which bioactive peptides are derived. ROS: reactive oxygens species.
Infections result from the interaction of the virus with host cell surface receptors (endosomal membrane fusion) or cytoplasmic membrane fusion, as illustrated in Figure 1. The internalization process of SARS-CoV-2 depends on TMPRSS2, a serine protease that cleaves and activates protein S.5 In this case, the viral S protein, consisting of spicules of glycoproteins, is cleaved into the S1 and S2 (a protein involved in a viral entry, via the endosome). The S1 is responsible for membrane binding by coupling to the extracellular juxtamembrane domain of the angiotensin-converting enzyme 2 (ACE2), expressed in different host tissues and lung cells.8 However, drugs interfering with angiotensinergic components do not appear to modify the course and clinical severity of COVID-19.9 Furthermore, to date, the literature lacks clinical evidence of any pharmacological approach for the angiotensinergic counterregulatory branch during COVID-19, although the involvement of ACE2 in SARS-CoV-2 infection is considered irrefutable10-12 and a promising therapeutic target.13 Research on these enzymes may provide important outcomes in the understanding of the COVID-19 pathophysiology.
In this context, it is necessary to highlight that the activity of serine proteases and S proteins, as mechanism for anchoring the SARS-CoV-2 to host cells, seems to be relevant .5,14 This action of the serine protease, a limiting step for the adhesion of the virus to the cell plasma membrane, allows its entry into the cell, suggesting that the greater aggressiveness of the SARS-CoV-2 may be a consequence of an increase in the direct entry of the virus into the cells, mediated by the serine protease TMPRSS2.15 In addition to entry via the S1, the virus can also enter through the fusion of some endosomal proteases, such as the Cathepsin L, and this turns this enzyme into a potential pharmacotherapy target. The upper part of Figure 1 outlines the viral structure and the hypothetical processes of the SARS-CoV-2 fusion and infection.5
The SARS-CoV-2 genome encodes 14 polyproteins Open Reading Frames (ORFs), responsible for replicating viral RNA.16 At the 5′ end, the largest gene ORF1ab is involved in the coding of 14 non-structural proteins. The 3’ end that harbors genes encoding accessory proteins (ORF3a, ORF6, ORF7a, ORF8 and ORF10) comprises structural protein genes that allow the entry to, and infection of, the host cell.17 The ORFs are responsible for the replication and virulence.16 The ORF7, for example, may be involved in the viral release, by reducing the expression of the tetherin protein (BST2 gene), which in regular conditions would block different enveloped viruses, such as the SARS-CoV-2, from leaving the host cell. When the virion sprouts out from the cell surface, the tetherin anchored to the cell membrane attaches to a new viral membrane, by hooking the virion to the host cell.18 Additionally, the ORF7a overexpression may result in apoptosis, via the caspase, besides blocking cell cycles at the G0 and G1 phases.19 These modifications in the early stage of cell maturation may impact hematopoiesis. In this sense, the development of pharmacological tools that effectively and directly inhibit the ORFs activity could be innovative and determinant in a better prognosis for those infected by SARS-CoV-2.
The SARS-CoV-2 infection, that evokes ORF-mediated cell damage, more specifically exerted by the ORF3a, activates nucleotide-binding oligomerization. According to Yap and collaborators, the ORF3a promotes the assembly of the inflammasome in a big molecular structure, generating a platform for the mass recruitment and activation of the caspase-1.20 As a mechanism that dramatically intensifies the pathogenesis of the disease, the activation of the inflammasome can trigger cell pyroptosis, a type of programmed cell death, characterized by the membrane rupture mediated by gasdermine D and the spontaneous release of the cytosolic content through the extracellular spaces.21 The pyroptosis-originated cell debris would trigger different effects. For example, the activation of the inflammasome and the pyroptosis, induced by the SARS-CoV-2 at the alveolar macrophages, may aggravate the pneumonia status and provoke acute respiratory distress syndrome and fever.20 The combination of leukocytosis and pyroptosis may be the main contributor to the cytokine storm observed in patients with COVID-19, which rapidly results in alarming excessive tissue inflammation, erythrocyte phagocytosis, organ failure and death.20,22 Therefore, a greater expression and activity of the ORFs could be the mechanism responsible for the chronification and worsening of the patient's clinical condition. Considering this evidence, it is possible to hypothesize that the SARS-CoV-2 infection-induced apoptosis, leading to lung damage, could share cellular and molecular mechanisms with the impairment of the blood cell dynamics and function during COVID-19.
Hematological implications of Sars-Cov-2 infection: pathophysiological erythrocytic protagonismThe literature still lacks consistent and conclusive findings on the effects of the SARS-CoV-2 infection on hematopoiesis. There is no study reporting on a causal correlation among the SARS-CoV-2 infection, changes in the bone marrow function and reductions in reticulocyte production, which increases the need for further research. The reticulocyte production results from the influences of several factors, including cytokines that are altered during COVID-19,23,24 whose maturity is reached within one or two days in the bloodstream circulation, as determined by the kidney secreted hormone erythropoietin.25 In this regard, it is expected to find hematopoietic changes in COVID-19 patients displaying renal complications related to the inflammatory status.26 Possible influences on the reticulocyte production may interfere with the erythrocyte replacement in the circulation,27 as this period is necessary to complete the synthesis and maturation of hemoglobin (Hb).23,25 A correlation among inflammatory signaling, erythropoietin, hematological outcomes and kidney diseases during COVID-19 is an important field that should be better explored.
With a biconcave morphology, anucleate and devoid of organelles, the erythrocytes are composed of the plasma membrane, cytoskeleton, hemoglobin and glycolytic enzymes.28 After the end of their lifespan (approximately 120 days), these cells are either removed by phagocytosis or destroyed by hemolysis in the spleen.29 Starting from the premise that the primordial erythrocyte activity is the gases transportation, disorders related to the morphophysiology, production, function and/or lifespan of red blood cells may result in tissue hypoxia due to the lack of oxygenation. The infection generated by the SARS-CoV-2 reduces the blood oxygen level, which can lead to rapid desaturation, although in some cases, dyspnea may be initially absent.30,31
According to Delphine Gérard and collaborators, patients with COVID-19 present mushroom cells and abnormal red cell morphology, with the presence of anisocytosis, spherocytosis, stomatocytes and polychromasia.32 These changes in the erythrocyte morphophysiology may be related to the imbalanced redox status found in infectious and inflammatory situations, as seen during COVID-19,33-35 which promotes oxidative stress in tissues by: i) elevating the levels of free radicals, including reactive oxygen species (ROS), and; ii) reducing the antioxidative enzymatic mechanisms, especially superoxide dismutase (SOD) and catalase (CAT). During metabolic processes, these free radicals mediate various biochemical reactions, including electron transfer for energy generation, activation of genes and defense mechanisms during the infection processes.32-37 However, oxidative damage is often associated either with the overproduction of free radicals and ROS, that attack important structures inside the cells, or with the downregulation in the antioxidant systems.37 Approximately 2% to 5% of the oxygen that is metabolized in the mitochondria is diverted to metabolic pathways that give rise to free radicals.38 This suggests that changes in the gases exchange at the cellular level, as a consequence of COVID-19-related alterations in erythrocyte dynamics, would contribute to these harmful metabolic routes. In addition, it is important to emphasize that inflammatory processes, such as those manifested during COVID-19, incisively participate in the inflammation-related oxidative stress due to the mechanism known as respiratory burst,39 in which phagocytes, especially macrophages, are active sources of free radicals and ROS, while exerting phagocytosis.40
Free radicals, such as the superoxide ion (O2−), hydroxyl radicals (OH) and ROS, such as hydrogen peroxide (H2O2), result from the univalent reduction of oxygen gas (O2). These chemical reactions are catalyzed by enzymes potentially altered by the SARS-CoV-2 infection and by iron,41 with the highlight for SOD, CAT and Glutathione.42 Despite not being a free radical, as it does have an unpaired electron in its last electron shell, the H2O2 is a highly reactive species, that can alter any nearby cell structure, proteins and organelles inside the cells. Unlike other free radicals, the H2O2 is long-lasting and can cross cell membranes with toxicity levels that can be increased 10,000x by the presence of iron. The binding of iron and copper to transferrin, ferritin and ceruloplasmin, as a mechanism of transportation, using and storage of these metals, is capable of preventing and/or minimizing the free radical generation reactions catalyzed by these metals. At the cytoplasm of liver cells, free iron (not bound to ferritin) is easily dissociated as an ion, which makes it catalytically active and available to participate in oxidation-reduction reactions and, consequently, in the generation of free radicals.33,43 Although iron is an essential metal for most biological organisms,34,35 it can modify the redox status and mediate the harmful oxidation of biomolecules, including DNA, proteins, lipids and others, when not tightly controlled.34 In the light of this possible free iron-dependent pathophysiology, it is worth hypothesizing that the SARS-CoV-2 infection would affect erythrocyte-related iron control mechanisms. This shall be supported by evidence that COVID-19 patients display an unfavorable redox status and an increase in serum ferritin, as a result of erythrocyte damage.43 The Figure 1 outlines the mechanisms underlying changes in the redox status in the hypothetical positive feedback loop resulting in the oxidative effects probably involved in erythrocyte and tissue damages.
The literature comprises data showing that the structural damage in the plasma erythrocyte membranes may result in shape loss, rheological changes,44 early lysis,28 excessive release of free ions and elevation of serum ferritin.45 These damages may be a consequence of the activity of non-structural proteins synthesized by the virus within blood cells, thus occasioning death. Extracellular hemoglobin, as a consequence of red cell death, is reported for enhancing oxidative reactions.46 Following on from these, therapeutic approaches attempting to reduce oxidative stress would be capable of minimizing damages on erythrocytes during COVID-19, thus increasing their lifespan and function, which would, by implication, result in more efficient oxygen transportation and delivery. Although antioxidant therapy was unable to shorten the duration of the symptoms,47 it should be considered for protecting blood components of those diagnosed with COVID-19.48 However, whether this choice would be effective in acting against the metabolic impairments caused by SARS-CoV-2 and whether this therapy would attenuate impacts on erythrocyte dynamics, remains to be fully investigated.
Changes in the activity and expression of plasma proteins closely related to erythrocyte, platelet and inflammatory vascular activities often accompany viral effects. The SARS-CoV-2 increases the α1-antitrypsin and acid α1-glycoprotein levels. These are plasma glycoproteins able to bind with lipophilic substances, such as hormones, medications and others.49 This increases by 3- to 4-fold are found in cases of acute or chronic inflammation, which highlight glycoproteins as candidates for inflammatory biomarking. Conversely, viral structural proteins reduce liver-synthetized α2-macroglobulin, whose activity is implicated in the inhibition of enzymes of the kallikrein-kinin system and complement system50 coagulation and fibrinolysis.6,51 This α2-macroglobulin decrease is also present in the COVID-19 inflammatory phase, making it another severity biomarker.52
The ceruloplasmin, that contains about 95% of the serum copper, also plays a protagonist role in the regulation of iron by performing its oxidation for subsequent incorporation by the transferrin (a complex called haptoglobin), which is irreversibly bound to the hemoglobin and prevents iron loss via kidney excretion.26,53,54 The correlation between free iron and the ceruloplasmin-haptoglobin ratio is considered a strong indicator of hemolysis28 and a potential tool for COVID-19 prognosis, as an increase in its value provides a clue to the acute phase of inflammatory diseases with a direct hemostatic repercussion.55 This idea is fully supported by data showing the incidence of iron-deficient anemia in patients who recovered from severe COVID-1956 and by the results indicating an association of alterations in anemia and iron metabolism biomarkers during the COVID-19 progression.45,54,57 The event cascades resulting from red cell compromise in severe COVID-1958 and the possible early hemolysis triggered by the SARS-CoV-2 infection involve hemopexin,59 which is responsible for free heme transport and recycle.60 Therefore, the physiological levels of hemopexin could not be enough to metabolize excess free iron in this infectious condition. The excessive level of iron alters the metabolism of the hemoglobin, myoglobin and of the iron-carrier known as transferrin.61 Such metabolic changes coexist with inflammatory processes involving chemotaxis, an oriented cellular recruitment of immune system components. In this manner, the hemopexin metabolism offers therapeutic targets and a prognosis for COVID-19.56,57,62
It is necessary to keep in mind that hemoglobin is a hemeprotein, whose structure is a globular tetramer formed by 4 units of the polypeptide chain: two α and two β units, attached by weak non-covalent bonds. Each protein or globin subunit has a heme covalently linked to iron at the center for oxygen binding. Heme is an important component of hemoglobin, composed of a porphyrin ring with iron (ferrous or Fe2 +), linked to 4 nitrogen atoms.63 Increases in ferritin production by the liver, in an attempt to reduce oxidative damage, may be a metabolic reactivity evoked by the COVID-19 inflammation-induced excessive free iron.57 All the cascade altering blood homeostasis that is a consequence of erythrocyte-related impairments in the iron metabolism may unravel potential biomarkers.56 Therefore, the attainment of a serum protein profile can provide important data on hemostasis and its possible COVID-19 prognostic correlations.
The SARS-CoV-2 surface glycoproteins and ORF polyproteins, specifically ORF8, can bind to porphyrin. At the same time, the ORF1ab, ORF10 and ORF3a can coordinate the attack on heme in the hemoglobin 1-β chain and dissociate iron to form porphyrin. The attack on the heme group will decrease functional hemoglobin levels, gradually affecting the oxygen and carbon dioxide transport. Consequently, lung cells may undergo extremely intense inflammatory toxicity due to their inability to exchange carbon dioxide and oxygen. This finding coincides with chest radiographic images showing ground-glass opacities likely found in diffuse lung disease caused by the SARS-CoV-2 infection.64,65
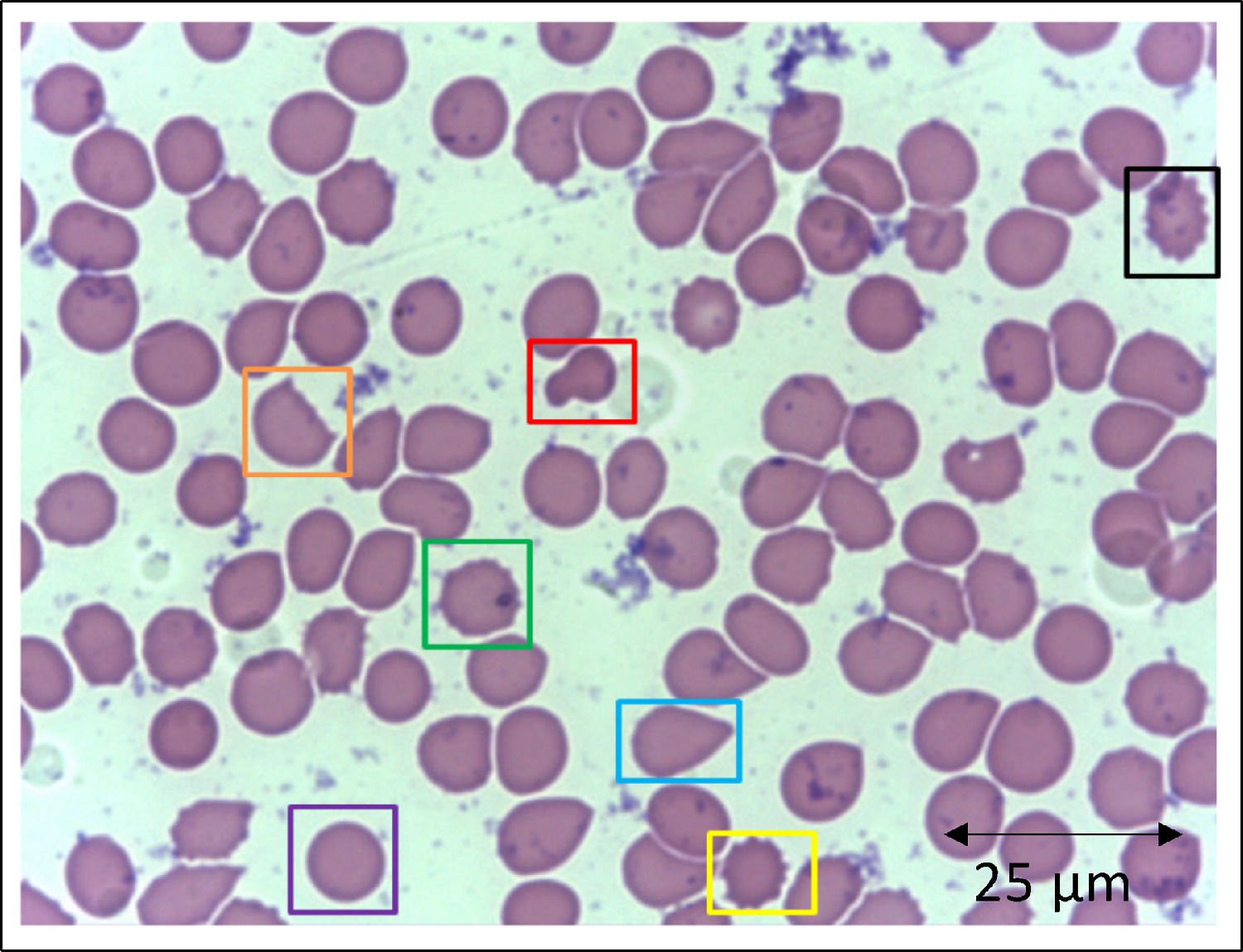
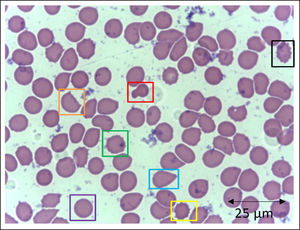
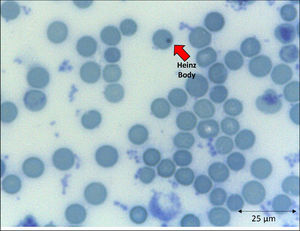
In addition to the changes in the red blood cell integrity, iron, and redox status, COVID-19 may be associated with a deficiency of the Band-3 protein prior to the appearance of mushroom erythrocytes and clamp cells, as well as Heinz bodies, that are intra-erythrocytic inclusions resulting from globin chains precipitation following hemoglobin oxidation, likely detected in cytopathological tests in COVID-19 patients. These bizarre erythrocytes can also be seen in bone marrow myelofibrosis and, occasionally, in nonspecific dyseritropoiesis.32,66 The following figure (Figure 2) is a blood sample taken from a critically ill COVID-19 patient showing hematological alterations that are also typically found during deficiency of the Band-3 protein in erythrocytes.32
Slide from a critically ill patient with COVID-19 admitted to an intensive care unit of a hospital. The slide details evidence of morphological alterations in red blood cells by the SARS-CoV-2 infection: red - mushroom red blood cells; green - red blood cells with the presence of Heinz bodies; blue - dacryocytes; purple - spherocytes; yellow - echinocytes (plasmolysis), and; black - acanthocytes; orange - keratocytes (bite cell). Staining method: Leishman. Magnification: 400X. Credits: Dr. Denise da Silva Pinheiro - LACES-ICB UFG.
The deficiency in the Band-3 protein in the red blood cell membrane often destabilizes multiprotein complexes,37 thus affecting the overall properties of the membrane morphology, mechanical stability and function. This can result in membrane detachment, with reduced cell deformability and loss of the biconcave morphophysiology. Such structural and biochemical changes can induce early cell removal from the circulation,36 thereby exacerbating the release of free radicals and other metabolites originated from the red blood cell death. Besides being recently pointed out as an alternative receptor for SARS-CoV-2,67 the Band-3 structure and function may be also changed by the oxidative and inflammatory status of COVID-19, as a feedback loop-based mechanism potentially feeding the destructive cascades that affect erythrocyte, endothelium, pneumocytes and others. In the erythrocyte, an interrelation among the internal cytoplasm fluidity with the membrane deformability and the protein complex of its surface ensures the functions of transporting O2 from the lungs and the CO2 removal from the tissues. This transport capacity depends on the conditions of circulatory adaptation, cytosolic hemoglobin and of a larger membrane polypeptide: the aforementioned Band-3. The Band-3 is primarily an ion exchange protein, also known as the anion exchanger 1, AE1 or HCO3−/Cl− exchanger.68,69 Considering that HCO3− is directly related to CO2 levels (therefore, it can determine respiratory acidosis) and that Band-3 is in charge of Cl−/HCO3− exchanges, a direct correlation of the Band-3 activity with an acid-base balance is undoubted. In addition, its interaction with lipids and other proteins constitute a cytoskeleton multiprotein complex that gives the erythrocytes mechanical and elastic properties,23,36,70 regulating blood viscosity. These erythrocyte membrane transport proteins are produced during the primordial stages of the erythroblasts.37 In light of this information, it is plausible to speculate that the presence of the SARS-CoV-2 in the early stages of hematopoiesis can modify the expression and activity of the Band-3 and this would contribute to the infectious pathophysiology. Further scientific studies are expected to provide evidence of these modifications.
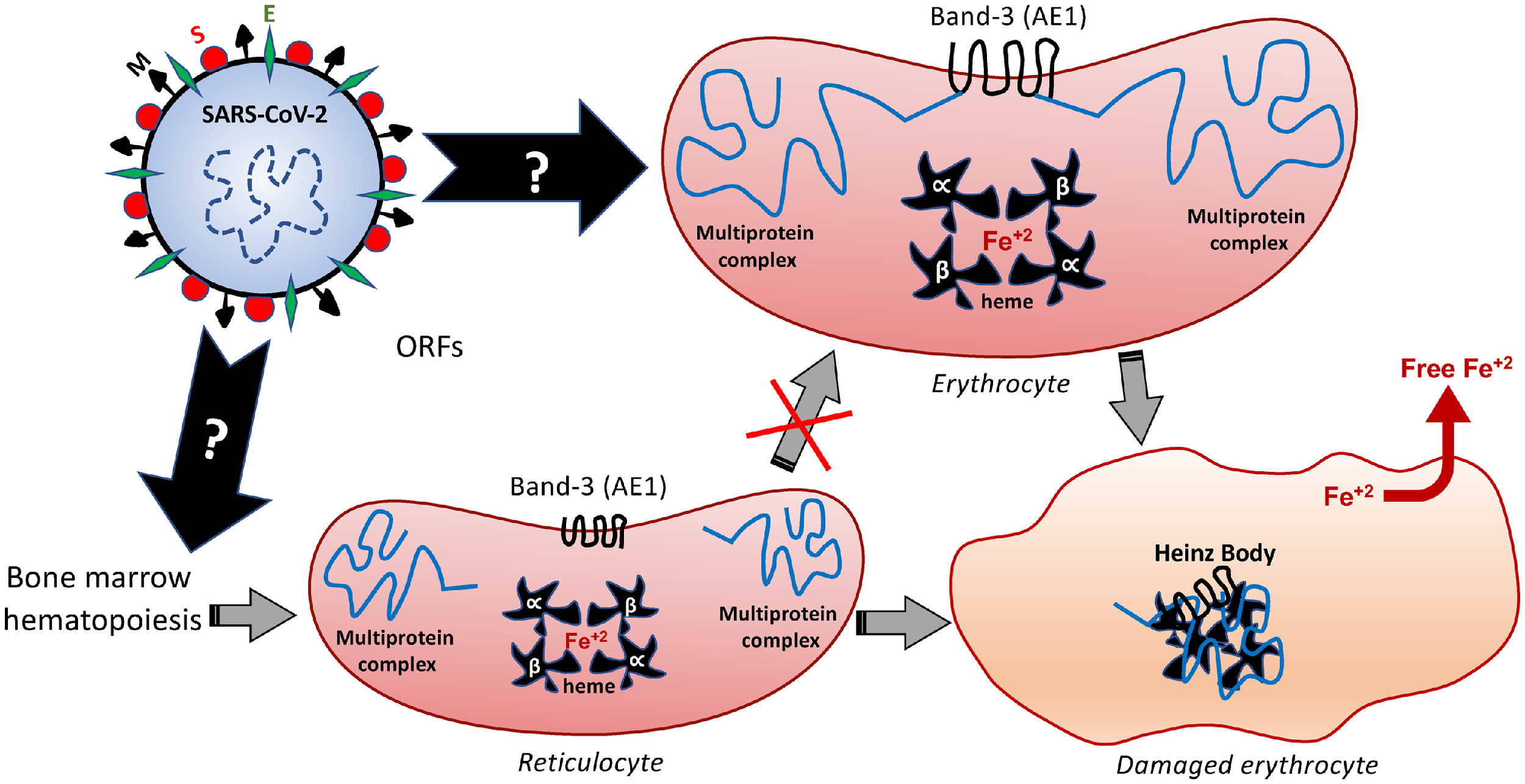
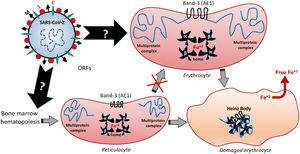
Among the different proteins anchored at the erythrocyte membrane, the cytoplasmic (intracellular) domain of the Band-3 is a major organizational center that interacts with many other peripheral proteins or ligands, such as ankyrin, protein 4.1, protein 4.2, aldolase, glyceraldehydes-3-phosphate dehydrogenase (G3PD), phosphofructokinase (PFK), deoxyhemoglobin, tyrosine kinase p72syk and hemichromes. This protein complex regulates the interaction of the cytoskeleton with glycolytic enzymes.68,71 Therefore, besides binding to the extracellular domain of the Band-3, similarly to that speculated for the ACE2 and endosomal mechanisms,5 the SARS-CoV-2 can also modify the structure and function of the intracellular Band-3 domain, resulting in intracellular disorganization and destabilization, even before the viral entry into the cell. Figure 3 comprises the molecular mechanisms involved in Band-3-related morphofunctional changes in erythrocytes.
Hypothetical mechanisms underlying the SARS-CoV-2-induced formation of Heinz Bodies as a clinical sign of hematological alteration that may occur through two possible routes, as represented by black arrows. The SARS-CoV-2 infection may affect the membrane Band-3-anchored multiprotein complex, thus resulting in erythrocyte damages. Furthermore, the SARS-CoV-2 infection may interfere with the early hematopoiesis processes in the bone marrow, resulting in the production and release of immature erythrocytes, the so-called reticulocytes. Therefore, this red blood cell maturation would be impaired by the viral infection, thus reducing the chances of producing a healthy erythrocyte, which may culminate in either reticulocyte death or its bizarre morphophysiological maturation.
Changes in the plasma levels of proteins involved in the COVID-19 pathophysiology can be useful in the prognosis, if measured immediately after diagnosis and frequently during the disease progression. The modulation of inflammation, coagulation and immune response by these proteins could be a mechanism underlying the hematological effects of COVID-19, thus allowing for precise correlations with treatments, severity, prognosis and patient survival. High levels of ferritin are indicative of hemolysis and are implicated in the COVID-19 severity since the patient's admission.72,73 These ferritin changes were reported57 as mediators of immune dysregulation, contributing to the cytokine storm.24 Therefore, assessing serum ferritin at different COVID-19 stages may clue important evidence on disease progression, consequently driving therapeutic approaches.
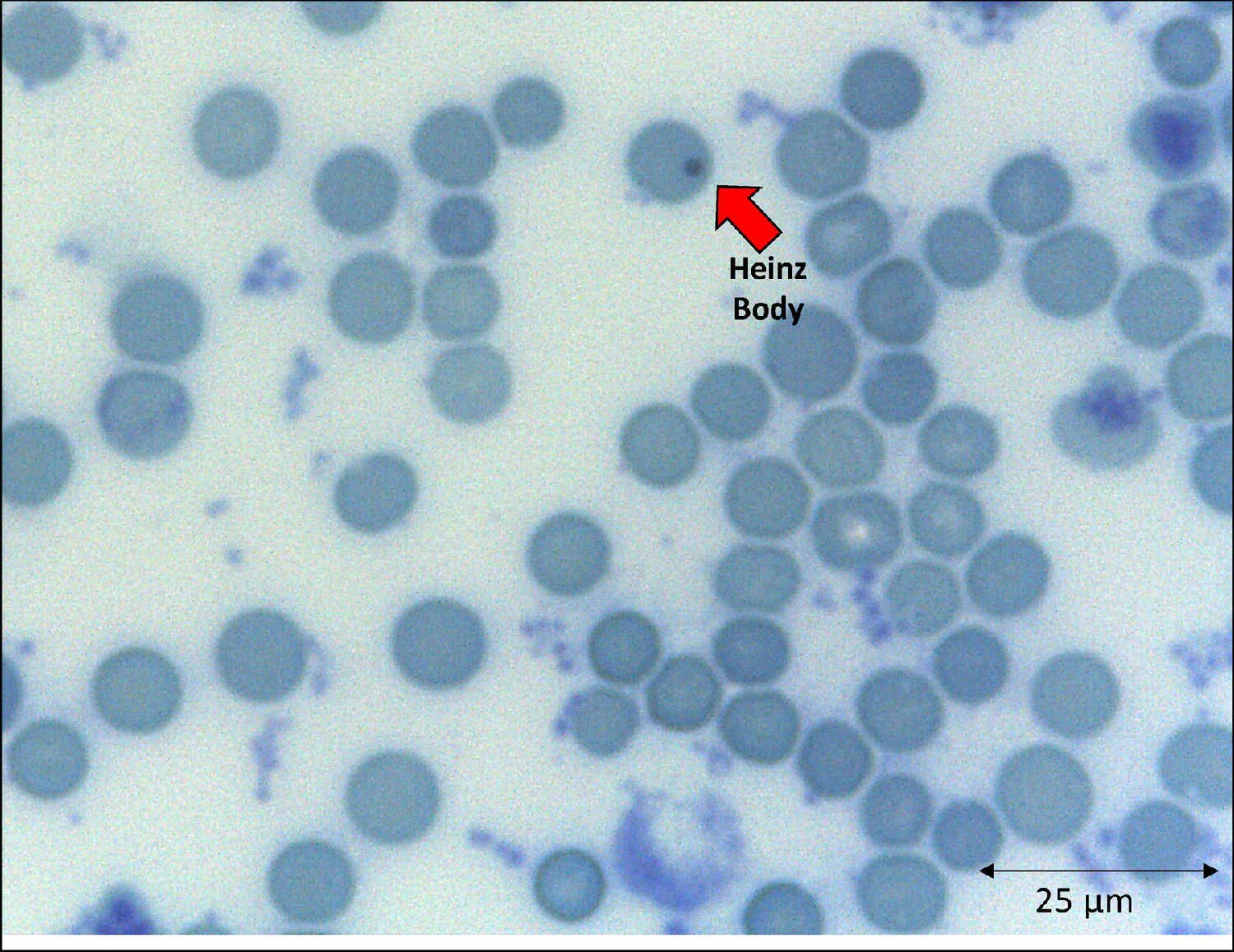
The emergence of unstable hemoglobin is another feasible hypothesis, as the SARS-CoV-2 may be entering the process of hemocytopoiesis, in addition to being highly mutagenic. Such mutagenicity results from the viral capacity to store genetic information also in RNA molecules, and this is the case with the SARS-CoV-2.74,75 Thus, the SARS-CoV-2 would cause mutations and generate polymorphisms. The gene expression resulting in the synthesis of the α-globin and β-globin chains65,76 highlights them as candidates for mutation detection studies. In hypothesis, mutations in erythrocyte proteins would result in inefficiency and structural kinetics, including hemoglobin. The structural alteration of the hemoglobin molecule, especially via the β chain, results in inefficiency and oxidation by excessive water entering the heme group.65,77 This process would be followed by the disintegration of the tetramer and this disaggregated polypeptide precipitates inside the erythrocytes as the Heinz body phenotype,76,77 as illustrated in Figure 4. However, despite the plausibility of the aforementioned hypotheses, the detection of polymorphisms in erythrocyte proteins and their causal relationship with mutations generated by the SARS-CoV-2 infection is another field necessitating future research.
With clinical and laboratory outcomes, the hematologic profile can predict the disease worsening or aid in the clarification of the pathophysiological mechanisms underlying viral attacks to different cells. The hemoglobin instability can be explained as a consequence of an abnormal connection between the alpha and beta globins, especially in the α1β1 contacts, and also the blockage of the side chain (SH radical) of the amino acid cysteine (Cis) that occupies position 93 of the β polypeptide chain.76,78 Decreases in the globin attraction to its heme groups are consequences of amino acid replacements that participate in the molecule stabilization and of changes in those aminoacidic residues spatially surrounding the heme group.79,80 If these processes occur in the β globin, reactive changes of side chains would be expected. The globin devoid of the heme is the main cause of the Heinz body formation, fixed on the erythrocyte membrane by double sulfur bonds.77 This bizarre protein arrangement affects cell permeability and, consequently, the cell function and lifespan.76
The degradation of erythrocytes may release the globin chains, which are metabolized by proteolytic enzymes81 and, as a result, the α-globin chain degradation releases hemopressins, whereas the β-globin chain is the substrate for the hemorphins production.81,82 These hemoglobin-derived bioactive peptides are transported in the bloodstream and are in charge of regulating several functions throughout the body.83 Some of these factors are the hemoglobin-derived peptides, which are systemically delivered from hemoglobin degradation, that occurs at the end of erythrocyte lifespan. Following the death of these cells, the activity of the enzymes ensures that hemoglobin is adequately hydrolyzed and peptides are released as a result of these enzymatic effects (Table 1). By assuming that the activity of these hemoglobin-derived peptides may be part of the puzzle resulting in homeostasis, it is plausible to propose that the acceleration of the erythrocyte degradation by the SARS-CoV-2 infection causes dysregulation in many systems.
Bioactive peptides derived from the α-globin and β-globin chain of hemoglobin.
Source: Da CRUZ, 2016.6
The hemorphins constitute the largest number of peptides released from the Hb degradation by enzymes, such as the aminopeptidase M,84 cathepsins B, D85,86 and E,87 prolyl oligopeptidase, neurolysin, dipeptidyl-peptidase IV,81 angiotensin-converting enzyme,84,88 lysosomal proteases,89 pepsin90,91 and pancreatic elastase.92 In healthy conditions, hemorphins have high stability in tissues78 and in human plasma86in vitro. It can also be metabolized in the cytosol of renal system cells in vitro,93 degraded by cathepsin B,89 dipeptidyl peptidase IV,94 prolyl oligopeptidase,93 neurolysin and endopeptidase.92 The involvement of cathepsin, lysosomal proteases and other peptidases in the pathophysiology of the SARS-CoV-2 infection78 supports the implication of this virus in the enzymatic processes leading to the erythrocyte death and subsequent dysregulation of different systems.
Hemorphins have several activities, such as: antinociception acting as opioid receptor agonists91,94,95; modulation on the activity of the calmodulin-dependent enzyme96; inhibition of encephalin degradation97 and angiotensin-converting-enzyme78; induction of central β-endorphins release,82 and; oxytocinergic activation.79 Hemoglobin-derived peptides also display antinociceptive91 and cardiovascular effects,98,99 increases spatial learning100,101 and cholinergic neurotransmission,102 modulates the pituitary-adrenal axis and reduces the activity of calcineurin.103 These peptides further regulate the synapses and release of classical neurotransmitters, neuropeptides and the adrenocorticotrophic hormone.104-106 Therefore, changes in the hemoglobin-derived peptide during COVID-19 and other diseases may interfere with several functions.
Although these studies mostly show the role of hemoglobin-derived peptides in physiological functions, it is likely that these peptides participate in the pathophysiology of some diseases,81,107 which are comorbidities representing the risk of becoming seriously ill during COVID-19.65,108 In addition, there is evidence of an increase in the level of hemorphins in the brain of patients with Alzheimer's disease109 and this may share pathophysiological aspects resulting in the cognitive sequelae in patients who recovered from COVID-19.110,111 Therefore, the questions that remain open are on whether: i) the SARS-CoV-2 would influence the activity of enzymes that degrade hemoglobin, and; ii) the SARS-CoV-2 would modify the structure and activity of hemoglobin-derived peptides and their bioavailability. Addressing these questions would open an unexplored field, providing information that would lead to the elaboration of new therapeutic strategies for diagnosis and treatments.
ConclusionIt is still uncertain how many SARS-CoV-2 variants will surface and how long it will be primarily responsible for worldwide deaths from infectious diseases. However, laboratory and therapeutic approaches in several areas, with emphasis on the erythrocyte changes, can contribute substantially to the reduction of the morbidity and mortality due to COVID-19. The body of evidence and a growing number of hypotheses support viral alteration of the erythrocyte membrane proteins prior to changes in cellular shape, function and final destruction. The possible clinical findings potentially linked to erythrocytic dysregulations, that are proposed throughout the current manuscript, necessitate being better addressed. The detection of the factors involved in the excessive release of the iron ions, free radicals and hemoglobin-derived peptides could unravel the puzzling combination of pathophysiological mechanisms, while providing a prognosis for the evolution of the COVID-19.
FundingOngoing research from all authors are supported by CNPq, CAPES, FAPEG and FAPEMIG. Michelle Mendonça is a recipient of CAPES PhD fellowship in the Graduation Program in Biological Sciences (Physiology and Pharmacology) of the Federal University of Goiás. Carlos Xavier - Chamada colaborativa FAPEG/FAPESP 2019 and CNPq (PQ-308156/2018-8).
This manuscript was written in honor of the volunteers who worked on the SARS-CoV-2 detection at the Clinical Analyses Laboratory (LACES-ICB) of the Federal University of Goiás – Brazil. In memoriam of Alan Eduardo Caldeira Custódio and Rainer Serra who died of COVID-19.