Hematopoietic stem cell transplantation is a curative treatment for many patients with hematological disorders. Donor–recipient genetic disparity, especially involving the human leukocyte antigen system is a critical factor for transplant outcome.
ObjectiveTo evaluate retrospectively donor characteristics and correlations with the occurrence of acute and chronic graft-versus-host disease, disease-free survival and overall survival in a Brazilian population submitted to allogeneic hematopoietic stem cell transplantation between 1994 and 2012 in a single center.
ResultsThree hundred and forty-seven consecutive transplantations were included. Related transplants (81.2%) were significantly more common than unrelated transplants (18.7%); donor and recipient median ages were 34 (range: 1–61) and 33 (range: 3–65) years respectively with donor HLAs being matched for 333 (95.9%) patients. Donor gender, cytomegalovirus status and ABO incompatibility did not influence the five-year overall survival. In univariate analyses, overall survival was negatively influenced by the presence of acute graft-versus-host disease (33% vs. 47%, respectively; p-value=0.04), unrelated transplant (41.5% vs. 50.9%, respectively; p-value=0.045) and donors aged over 40 years (41% vs. 52%, respectively; p-value=0.03). Older donors were associated with a higher rate of acute (52% vs. 65.8%; p-value=0.03) and chronic graft-versus-host disease (60% vs. 43%, respectively; p-value=0.015). In multivariate analyses, acute graft-versus-host disease [relative risk (RR): 1.8; 95% confidence interval (CI): 1.1–29; p-value=0.008] and older donors (RR: 1.6; 95% CI 1.11–2.24; p-value=0.013) were associated with higher transplant-related mortality.
ConclusionsIn transplant patients, to have a donor older than 40 years of age seems to significantly increase the incidence of acute and chronic graft-versus-host disease and transplant-related mortality with no impact on disease-free survival and overall survival. In spite of the rather small cohort of patients, these findings are similar to what is described in the literature suggesting that a younger donor should be chosen whenever possible.
Hematopoietic stem cell transplantation (HSCT) is a curative treatment for many patients with hematologic disorders.1,2 Its goal is to replace both the immune and the hematopoietic systems with healthy hematopoietic stem cells obtained from a human leukocyte antigen (HLA) compatible donor.3 Genetic disparity between donor and recipient, especially at HLA loci, is a critical factor for the outcome of HSCT.4
Despite advances in genetic characterization, immunosuppressive drugs and supportive care, acute and chronic graft-versus-host disease (GVHD) remain significant causes of morbidity and mortality after HSCT.5,6 In addition to genetic disparities and GVHD, disease status at transplant, source of stem cells, conditioning regimens and infectious complications are associated to HSCT outcome. Some other donor-related aspects, such as gender, age, cytomegalovirus (CMV) serological status and ABO incompatibility may also be associated with HSCT outcomes with their individual roles having been explored with variable results.7–10 In spite of pre-emptive treatment, the reactivation of CMV disease is still an important cause of morbidity and mortality.11
HSCT is being increasingly performed in over 50-year-old individuals due to the development of reduced intensity conditioning (RIC) regimens.12 As a consequence, older related compatible donors are also being accepted and the regenerative capacity of hematopoietic stem cells (HSC) and possible comorbidities are becoming issues, as recent studies have demonstrated that increased donor age may be a risk factor for acute and chronic GVHD.13
Currently, about 30–50% of HSCTs are performed with ABO incompatibility.14 It is well established that ABO incompatibility increases the risk of hemolytic reactions; however, according to recent data, it does not change the outcome of HSCT.8,15
This study evaluated the influence of donor characteristics such as age, gender, CMV status, cell source, ABO compatibility and type of donor (matched related – MRD or matched unrelated – MUD) on the outcome of HSCT in a cohort of 347 patients transplanted at the Hospital de Clinicas in Porto Alegre, southern Brazil. We wanted to know whether such characteristics would predict outcomes in this Latin American cohort of patients transplanted in a single center.
MethodsThree hundred and forty-seven patients submitted to allogeneic HSCT from January 1994 to December 2012 at a single center were evaluated retrospectively. The donor and recipient ages, donor gender, CMV status, ABO compatibility, type of donor (matched related, and matched unrelated) and patient's disease status were correlated with the occurrence of acute and chronic GVHD, disease-free survival (DFS) and overall survival (OS).
All patients had given their informed written consent at the time of the procedure and the study was approved by local Ethics Committee. Advanced disease status at HSCT was defined as refractory disease, second or more remission to malignant disease or more than one year of diagnosis of benign disease.
Donor selection and HLA typingHLA Class I (A, B, C) and Class II (DQ and DR) typing of patients and related donors was performed by conventional serology until 2000 and low resolution DNA-based typing thereafter. For unrelated donor HSCT, performed in this center since 2005, high resolution HLA typing was performed for 6/6 matches up to 2008 and 8/8 or 10/10, thereafter.
Conditioning regimensStandard myeloablative conditioning (MAC) consisted of 14–16mg/kg oral busulfan (BU) plus 2×60mg/kg cyclophosphamide (CY) or CY (2×60mg/kg) plus total body irradiation (12Gy fractioned dosage). The RIC regimens utilized were as follows: BU 8–10mg/kg PO plus 90–120mg/m2 fludarabine (Flu), or Flu (120mg/m2) plus 140mg/m2 melphalan or CY 60mg/kg. Patients submitted to MUD transplants also received rabbit thymoglobulin (7–14mg/kg).
Graft-versus-host disease prophylaxisPatients on MDR and MAC regimens received cyclosporin A (CYA) (3mg/kg IV) starting on Day −1 and an additional short course of methotrexate (MTX) (15mg/m2) on Day +1 and 10mg/m2 on Days +3, +6 and +11. For those undergoing MUD transplants, tacrolimus (0.05mg/kg IV) was associated with a short course of MTX. For RIC, GVHD prophylaxis was achieved with 2g mycophenolate mofetil daily (Day +1 to Day +30) plus CYA 3mg/kg PO starting on Day −2. When umbilical cord HSC was the source, the short course of MTX was not utilized.
EngraftmentEngraftment was defined as peripheral granulocyte counts above 500/μ-L for three consecutive days. A primary engraftment failure or rejection was defined when engraftment was not obtained in patients who survived more than 28 days after transplantation. The rate of engraftment failure was calculated at Day 100 after the procedure.
Supportive careAll patients were kept in a protective environment with laminar high efficiency particulate air (HEPA) filters. Prophylactic acyclovir, fluconazole, and sulfamethoxazole plus trimethoprim were routinely administered to all patients. Weekly CMV monitoring was carried out by qualitative DNA-polymerase chain reaction (PCR) up to 2005 and by antigenemia assaying thereafter. Pre-emptive 10mg/kg ganciclovir was started after two consecutive positive PCR results or one positive cell in the antigenemia assay. All blood products were irradiated and filtered. Minimum values were set to trigger red blood cell transfusions to maintain hemoglobin greater than 7g/dL and platelets to maintain the platelet count higher than 20×109/L according to the hospital transfusion committee norms.
Neutropenic fever was treated with broad-spectrum antibiotics according to our hospital protocols which were based on our microbiological sensitivity profile and on Infection Diseases Society of America (IDSA) Guidelines.16,17
Statistical analysisThe characteristics of patients and donors are expressed as medians and range for continuous variables and frequencies for categorical variables. The primary endpoint of the analysis was OS and secondary endpoints were the incidence of acute and chronic GVHD, DFS and transplant-related mortality (TRM). Acute GVHD was staged and graded (Grade 0-IV) by the number and extent of organ involvement. OS was estimated using the Kaplan–Meyer method. Comparison of curves was calculated using the log-rank test. Categorical data were compared using the Chi-square test. The following variables were included in the analyses: age and gender of patients and donors, patient–donor gender combination, patient and donor CMV-serological status, stem cell source [bone marrow (BM), peripheral blood stem cell (PBSC) and cord blood stem cell (CBSC)], dose of CD34+ cells, MAC vs. RIC, MUD vs. MRD, and patient's disease status. Factors with p-value <0.2 were included in multivariate analyses. The Cox proportional hazard regression model was used for multivariate analysis. To evaluate the influence of donor's age in transplant outcomes, a cut-off value was set at 40 years based on the literature and on the somewhat lower age of patients and donors in our cohort.7,9,13,18 This study was approved by the Ethics Committee of HCPA and the data were analyzed anonymously according to the Declaration of Helsinki for human studies.
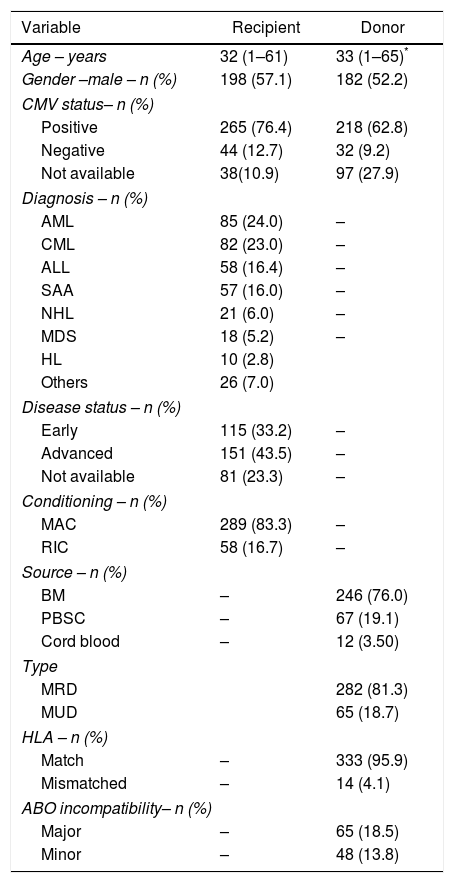
ResultsA total of 347 patient–donor pair charts were reviewed, comprising all patients submitted to allogeneic HSCT in the study center from 1994 to 2012. The characteristics of patients and donors are summarized in Table 1. The median age of patients was 32 (range: 1–61) years, 198 (57.1%) were male, a MAC regimen was utilized in 289 (83.3%), and the HSC source was BM in 246 (76%). The majority of patients had a malignant condition: 85 (24%) had acute myeloid leukemia, 58 (16.4%) had acute lymphoblastic leukemia, 82 (23%) had chronic myeloid leukemia, 18 (5.2%) had myelodysplastic syndrome, 21 (8.8%) had lymphomas, 57 (16%) had aplastic anemia and 26 (7%) had other conditions. Disease status was advanced (beyond second remission) in 151 (43.5%) patients. CMV serological status was positive in 265 (85.8%) and 218 (87.2%) patients and donors, respectively. The median age of donors was 33 (range: 1–65) years, 182 (52.2%) were male and 282 (81.3%) were matched-related.
Characteristics of patients and donors of 347 hematopoietic stem cell transplants.
| Variable | Recipient | Donor |
|---|---|---|
| Age – years | 32 (1–61) | 33 (1–65)* |
| Gender –male – n (%) | 198 (57.1) | 182 (52.2) |
| CMV status– n (%) | ||
| Positive | 265 (76.4) | 218 (62.8) |
| Negative | 44 (12.7) | 32 (9.2) |
| Not available | 38(10.9) | 97 (27.9) |
| Diagnosis – n (%) | ||
| AML | 85 (24.0) | – |
| CML | 82 (23.0) | – |
| ALL | 58 (16.4) | – |
| SAA | 57 (16.0) | – |
| NHL | 21 (6.0) | – |
| MDS | 18 (5.2) | – |
| HL | 10 (2.8) | |
| Others | 26 (7.0) | |
| Disease status – n (%) | ||
| Early | 115 (33.2) | – |
| Advanced | 151 (43.5) | – |
| Not available | 81 (23.3) | – |
| Conditioning – n (%) | ||
| MAC | 289 (83.3) | – |
| RIC | 58 (16.7) | – |
| Source – n (%) | ||
| BM | – | 246 (76.0) |
| PBSC | – | 67 (19.1) |
| Cord blood | – | 12 (3.50) |
| Type | ||
| MRD | 282 (81.3) | |
| MUD | 65 (18.7) | |
| HLA – n (%) | ||
| Match | – | 333 (95.9) |
| Mismatched | – | 14 (4.1) |
| ABO incompatibility– n (%) | ||
| Major | – | 65 (18.5) |
| Minor | – | 48 (13.8) |
* Donor age <40 (n=117–37.7%) and >40 (n=193–62.3%).
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; SAA: severe aplastic anemia; CML: chronic myeloid leukemia; MDS: myelodysplastic syndrome: NHL: non-Hodgkin lymphoma; HD: Hodgkin's lymphoma; CMV: cytomegalovirus; RIC: reduced intensity conditioning; MAC: myeloablative conditioning; BM: bone marrow; PBSC: peripheral blood stem cells; MRD: matched related donor; MUD: matched unrelated donor; Others: immunodeficiency, sickle-cell anemia, myelofibrosis.
The five-year OS for the whole group was 49.1% [95% confidence interval (CI): 41–54%]. Engraftment occurred in 317 patients (92.4%) with the mean time to engraft being 19 days (range: 8–45). The mean CD34+ cell dose was 3.4×106/kg (range: 1–34×106/kg). ABO incompatibility existed in 113 (32.3%) transplants with 65 (18.5%) having major and 48 (13.8%) minor incompatibility. Compared with 19.5 days for engraftment of patients without incompatibility, major and minor incompatibility did not influence engraftment with 20.3 days (p-value=0.293) and 18.6 days (p-value=0.100), respectively. There were no differences in time to engraftment for younger (19.7 days) and older donors (18.7 days; p-value=0.063).
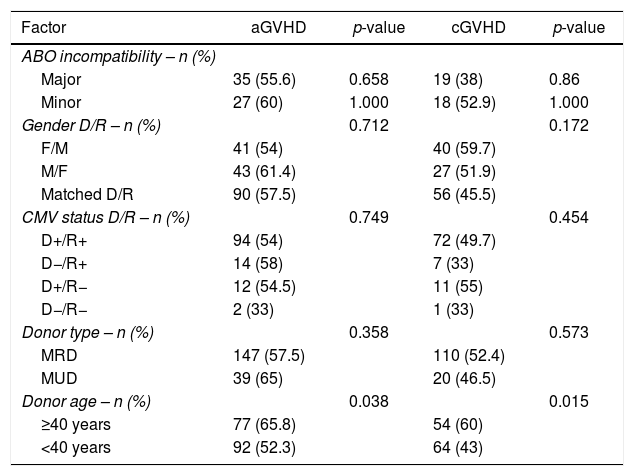
Acute GVHD (Grades I–IV) was present in 185 (62.5%) patients and chronic GVHD in 131 (50.4%). There were no differences in the cumulative incidence of acute GVHD for MRD vs. MUD (147–57.5% and 39–65%, respectively; p-value=0.358) or for chronic GVHD (110–52.6% and 20–46.7%, respectively; p-value=0.573). Acute and chronic GVHD were significantly more common when donors were older than 40 years. Acute GVHD occurred in 77 (65.8%) recipients from older (>40 years) donors and in 92 (52%) from younger donors (p-value=0.03). Chronic GVHD occurred in 54 (60%) recipients from older (>40 years) donors and in 64 (43%) recipients from younger donors (p-value=0.015). ABO incompatibility, donor gender, MRD or MUD, and CMV serological status had no impact on the occurrence of acute or chronic GVHD (Table 2).
Univariate analyses of ABO incompatibility, donor gender, cytomegalovirus status, donor type and age in 347 transplanted patients in relation to acute or chronic graft-versus-host disease.
| Factor | aGVHD | p-value | cGVHD | p-value |
|---|---|---|---|---|
| ABO incompatibility – n (%) | ||||
| Major | 35 (55.6) | 0.658 | 19 (38) | 0.86 |
| Minor | 27 (60) | 1.000 | 18 (52.9) | 1.000 |
| Gender D/R – n (%) | 0.712 | 0.172 | ||
| F/M | 41 (54) | 40 (59.7) | ||
| M/F | 43 (61.4) | 27 (51.9) | ||
| Matched D/R | 90 (57.5) | 56 (45.5) | ||
| CMV status D/R – n (%) | 0.749 | 0.454 | ||
| D+/R+ | 94 (54) | 72 (49.7) | ||
| D−/R+ | 14 (58) | 7 (33) | ||
| D+/R− | 12 (54.5) | 11 (55) | ||
| D−/R− | 2 (33) | 1 (33) | ||
| Donor type – n (%) | 0.358 | 0.573 | ||
| MRD | 147 (57.5) | 110 (52.4) | ||
| MUD | 39 (65) | 20 (46.5) | ||
| Donor age – n (%) | 0.038 | 0.015 | ||
| ≥40 years | 77 (65.8) | 54 (60) | ||
| <40 years | 92 (52.3) | 64 (43) | ||
F: female; M: male; D: donor; R: recipient; MDR: matched related donor; MUD: matched unrelated donor; aGVHD: acute graft-versus-host disease; cGVHD: chronic graft-versus-host disease.
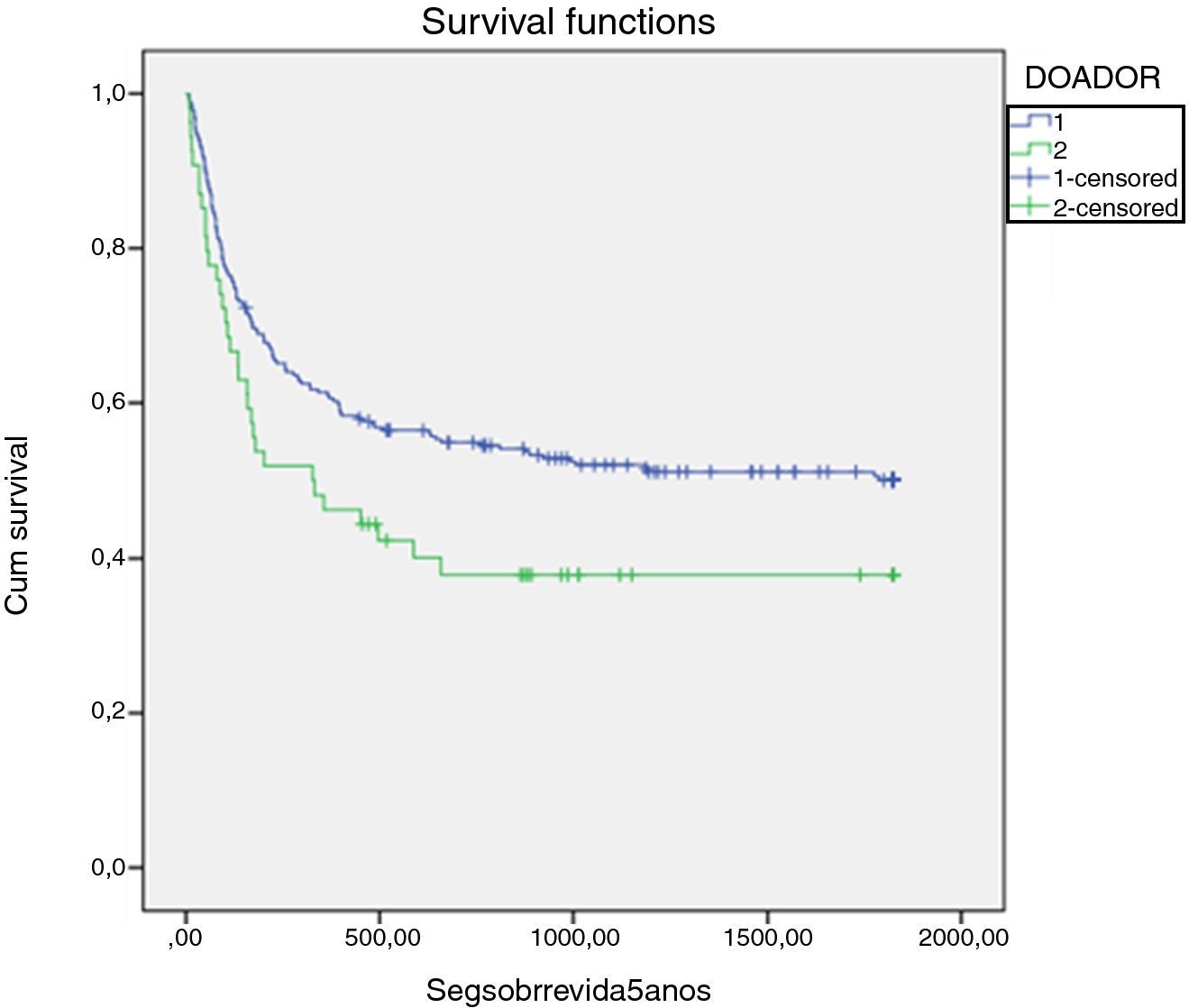
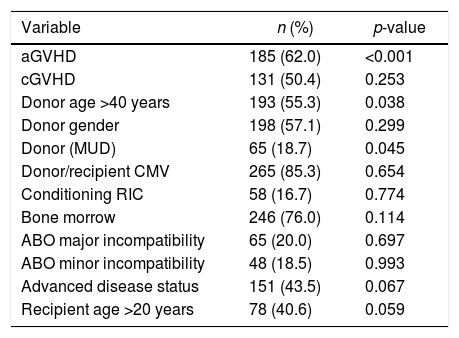
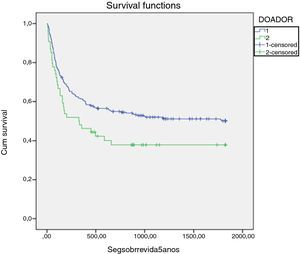
Results of the univariate analysis of the impact of relevant variables on OS are summarized in Table 3. The five-year OS of all transplanted patients according to the donor's age revealed significantly greater survival for recipients of younger donors (<40 years) (52% vs. 41%; p-value=0.038). In this analysis, the five-year OS was negatively influenced by acute GVHD, with 40.3% of patients with acute GVHD alive at that time-point vs. 69.1% without acute GVHD (p-value=0.001). A similar difference in OS was observed for the donor type, with 41.5% MUD vs. 50.9% MRD transplanted recipients surviving at least five years (p-value=0.045; Figure 1).
Univariate analysis by log-rank test of the impact of the variables on overall survival after 347 hematopoietic stem cell transplants.
| Variable | n (%) | p-value |
|---|---|---|
| aGVHD | 185 (62.0) | <0.001 |
| cGVHD | 131 (50.4) | 0.253 |
| Donor age >40 years | 193 (55.3) | 0.038 |
| Donor gender | 198 (57.1) | 0.299 |
| Donor (MUD) | 65 (18.7) | 0.045 |
| Donor/recipient CMV | 265 (85.3) | 0.654 |
| Conditioning RIC | 58 (16.7) | 0.774 |
| Bone morrow | 246 (76.0) | 0.114 |
| ABO major incompatibility | 65 (20.0) | 0.697 |
| ABO minor incompatibility | 48 (18.5) | 0.993 |
| Advanced disease status | 151 (43.5) | 0.067 |
| Recipient age >20 years | 78 (40.6) | 0.059 |
aGVHD: acute graft-versus-host disease; cGVHD: chronic graft-versus-host disease; CMV: cytomegalovirus; MUD: matched unrelated donor; MRD: matched related donor; RIC: reduced intensity conditioning; MAC: myeloablative conditioning; PBSC: peripheral blood stem cells.
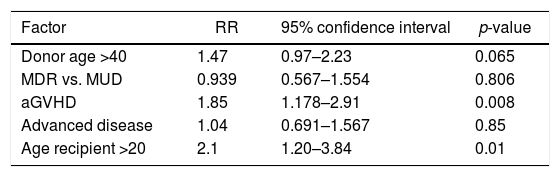
In multivariate Cox regression analysis (Table 4) only donor age [relative risk (RR): 1.68; 95% CI: 1.11–2.54; p-value=0.013] and acute GVHD (RR: 1.8; 95% CI: 1.17–2.91; p-value=0.008) had significant negative impacts on the five-year OS. In order to exclude a possible positive influence of the age of children/younger recipients on the general outcome, the recipient's age was included in multivariate analysis. This led to a reduction of the RR from 1.68 to 1.47 and a loss of significance of donor age as a factor influencing the five-year OS (95% CI: 0.97–2.23; p-value=0.065).
Multivariate Cox regression analysis for overall survival.
| Factor | RR | 95% confidence interval | p-value |
|---|---|---|---|
| Donor age >40 | 1.47 | 0.97–2.23 | 0.065 |
| MDR vs. MUD | 0.939 | 0.567–1.554 | 0.806 |
| aGVHD | 1.85 | 1.178–2.91 | 0.008 |
| Advanced disease | 1.04 | 0.691–1.567 | 0.85 |
| Age recipient >20 | 2.1 | 1.20–3.84 | 0.01 |
aGVHD: acute graft-versus-host disease; MRD: matched related donor; MUD: matched unrelated donor; RR: relative risk.
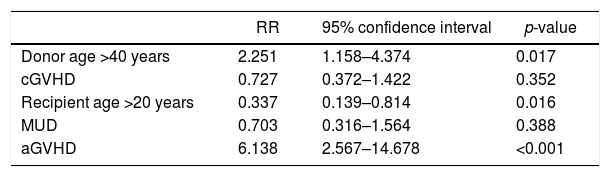
For the entire group of 347 patients, the estimated five-year TRM was 43.8% (95% CI: 38.1–49.4). The median follow-up of surviving patients was 76 months (range: 4–152 months). In univariate analysis, recipients of older donors had a higher TRM rate compared to those of younger donors: 52.9% vs. 36.4%, respectively (p-value=0.018). The presence of acute GVHD led to an increase in TRM (53% vs. 22%; p-value=0.003), but the effect of chronic GVHD was not significant (27.5% vs. 16.8%; p-value=0.145). Younger (<20-year-old) recipients had a lower TRM (29.9%) compared to older recipients (51.3%; p-value=0.02). By Cox regression, receiving a graft from a donor older than 40 years of age, the presence of acute GVHD, and age older than 20 years were independent risk factors for TRM (Table 5).
Multivariate Cox regression for transplant-related mortality.
| RR | 95% confidence interval | p-value | |
|---|---|---|---|
| Donor age >40 years | 2.251 | 1.158–4.374 | 0.017 |
| cGVHD | 0.727 | 0.372–1.422 | 0.352 |
| Recipient age >20 years | 0.337 | 0.139–0.814 | 0.016 |
| MUD | 0.703 | 0.316–1.564 | 0.388 |
| aGVHD | 6.138 | 2.567–14.678 | <0.001 |
MUD: matched unrelated donor; aGVHD: acute graft-versus-host disease; RR: relative risk.
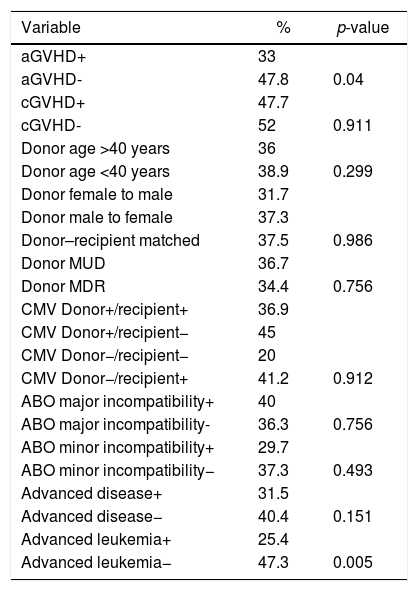
DFS was evaluated by the log rank test for the 268 patients transplanted for malignant diseases. There was no difference in the five-year DFS for the presence of major or minor ABO incompatibility (36.3% vs. 40%; p-value=0.75, and 29.7% vs. 37.3%; p-value=0.493, respectively). This was also true for gender mismatch between donor and recipient (31.7% vs. 37.3% vs. 37.5% for female to male, male to female and matched donor/recipient, respectively; p-value=0.986), for MRD and MUD transplants (36%.7 vs. 34%; p-value=0.089), and donor age (33% vs. 38.9% for younger and older than 40 years old, respectively; p-value=0.299).
In univariate analysis, acute GVHD had a negative influence on DFS (33% vs. 47.8%; p-value=0.004) but the presence of chronic GVHD had no effect (47.7% vs. 52%; p-value=0.911). Only in the acute leukemia group (n=143), advanced disease was a factor to reduce DFS (25.4% vs. 47.3; p-value=0.005). As can be seen in Table 6, the presence of acute GVHD and advanced disease for the acute leukemia patient group was significantly associated with lower DFS (p-value=0.004 and p-value=0.005, respectively). By Cox regression multivariate analysis, only acute GVHD continued with a negative influence on DFS.
Univariate analysis by log-rank test of disease free survival.
| Variable | % | p-value |
|---|---|---|
| aGVHD+ | 33 | |
| aGVHD- | 47.8 | 0.04 |
| cGVHD+ | 47.7 | |
| cGVHD- | 52 | 0.911 |
| Donor age >40 years | 36 | |
| Donor age <40 years | 38.9 | 0.299 |
| Donor female to male | 31.7 | |
| Donor male to female | 37.3 | |
| Donor–recipient matched | 37.5 | 0.986 |
| Donor MUD | 36.7 | |
| Donor MDR | 34.4 | 0.756 |
| CMV Donor+/recipient+ | 36.9 | |
| CMV Donor+/recipient− | 45 | |
| CMV Donor−/recipient− | 20 | |
| CMV Donor−/recipient+ | 41.2 | 0.912 |
| ABO major incompatibility+ | 40 | |
| ABO major incompatibility- | 36.3 | 0.756 |
| ABO minor incompatibility+ | 29.7 | |
| ABO minor incompatibility− | 37.3 | 0.493 |
| Advanced disease+ | 31.5 | |
| Advanced disease− | 40.4 | 0.151 |
| Advanced leukemia+ | 25.4 | |
| Advanced leukemia− | 47.3 | 0.005 |
aGVHD: acute graft-versus-host disease; cGVHD: chronic graft-versus-host disease; MRD: matched related donor; MUD: matched unrelated donor; CMV: cytomegalovirus.
In the last decade, much has been done to increase the efficacy of HSCT with the use of DNA-based high resolution HLA typing, the emergence of non-myeloablative conditioning regimens and better clinical support.19 As a consequence, the number of MUD transplants is increasing all over the world, and although acute and chronic GVHD rates are higher, survival outcomes are similar to those observed with MRD transplants.20 The use of RIC regimens has increased the ability to transplant older patients with a consequent increase in donor age in the MRD scenario. Although still controversial, the age of the donor and female to male transplants have been shown to have an impact on GVHD and survival.7,21
In 6978 MUD transplants, a donor age older than 45 years increased GVHD and had a negative impact on OS.21 On the other hand, donor age older than 50 years did not affect the outcomes of MUD RIC transplants or those of patients submitted to PBSC transplants although a cutoff age of 60 years was utilized for the latter group.22
In the current group of patients, grafts from donors older than 40 years of age were significantly correlated to the occurrence of acute (p-value=0.038) and chronic GVHD (p-value=0.015), in accordance with what was described in 6978 patients submitted to MUD transplants using a similar donor cutoff age (39 years).7 The fact that in the current study, most patients were submitted to MRD transplants, bone marrow was the major source of HSC, and most donor–patient pairs were CMV positive, precludes comparison. We could speculate, however, that setting a higher cutoff age might overshadow the influence of much younger donors on HSCT outcomes.
Donor gender and ABO incompatibility did not influence GVHD, DFS or OS in this population.
The lack of impact of the donor gender on this cohort of patients could be attributed to a tendency to choose, whenever possible, male donors since female to male transplants have been shown to increase acute GVHD,23 chronic GVHD, particularly of multiparous woman7,24 and longer immunosuppression.25 Of note, DFS was described as independently better for female to male pairs, suggesting that the graft-versus-leukemia effect was stronger in this combination.26
Major or minor ABO incompatibility, although observed in 32.3% of the present cohort, did not have any impact on GVHD, TRM, DFS or OS. Randolph et al.26 described a negative impact of ABO incompatibility (major, minor or bi-directional) on OS, and Seebach et al.27 found an increased risk of acute GVHD in bi-directional ABO incompatibility. A metanalysis designed to overcome the heterogeneity of ABO incompatibility and HSCT in different studies was not able to find an influence of such disparities on GVHD or OS. However, when only the patients submitted to MUD transplants were analyzed the authors observed a negative impact on OS of minor and bi-directional ABO incompatibilities.8 Also in the setting of MUD transplants, Kimura et al.28, on analyzing the Japanese registry, were able to show in a cohort of 5549 patients that major or minor incompatibility negatively affected TRM and OS without influencing DFS. The number of MUD transplants in this study (18.4% or 65 of 347) precludes such analysis.
Finally, over 80% of the patients and donors in this study tested positive for CMV precluding the analysis of the impact of CMV status on transplant outcome.
In conclusion, in this cohort of 347 patients, transplants from donors older than 40 years of age increases the incidence of acute and chronic GVHD and TRM significantly, with no impact on the engraftment rate, DFS or OS. Donor gender (female donor to male recipient) as well as ABO incompatibility did not have any influence on the transplant outcome. To our knowledge, this is the first study on the influence of the characteristics of donors on allogeneic HSCT outcomes performed in a Latin American cohort of patients.
Conflicts of interestThe authors declare no conflicts of interest.
The study received financial support from the Research and Event Incentive Fund of Hospital de Clínicas de Porto Alegre (FIPE-HCPA).