Diffuse large B-cell lymphoma (DLBCL) represents for 30–40% of the cases of non-Hodgkin lymphoma (NHL). Initial presentation of DLBCL in the leukemic phase is relatively unusual.1 We report a rare case of a 65-year-old man with DLBCL in the leukemic phase.
Case reportA 65-year-old man presented to the Medical Oncology Department with complaints of low-grade fever and swelling on the left side of his neck which he had noticed 2 weeks before and which was gradually progressive. He also complained of malaise and easy fatigability. On physical examination, the patient was pale and febrile (100°F). The left cervical lymph node at level IB was enlarged, measuring 3cm×3cm in diameter. The swelling was firm, non-tender, mobile and discrete. There was an absence of pain, discharge, ulceration or difficulty in chewing. Bilateral enlargement of the cervical lymph nodes at levels IB, II, III and V were observed, with axillary lymphadenopathy, the maximum size being 3cm×3cm in diameter, at left cervical lymph node level IB. Chest and cardiac examination were unremarkable and the abdominal examination showed moderate splenomegaly.
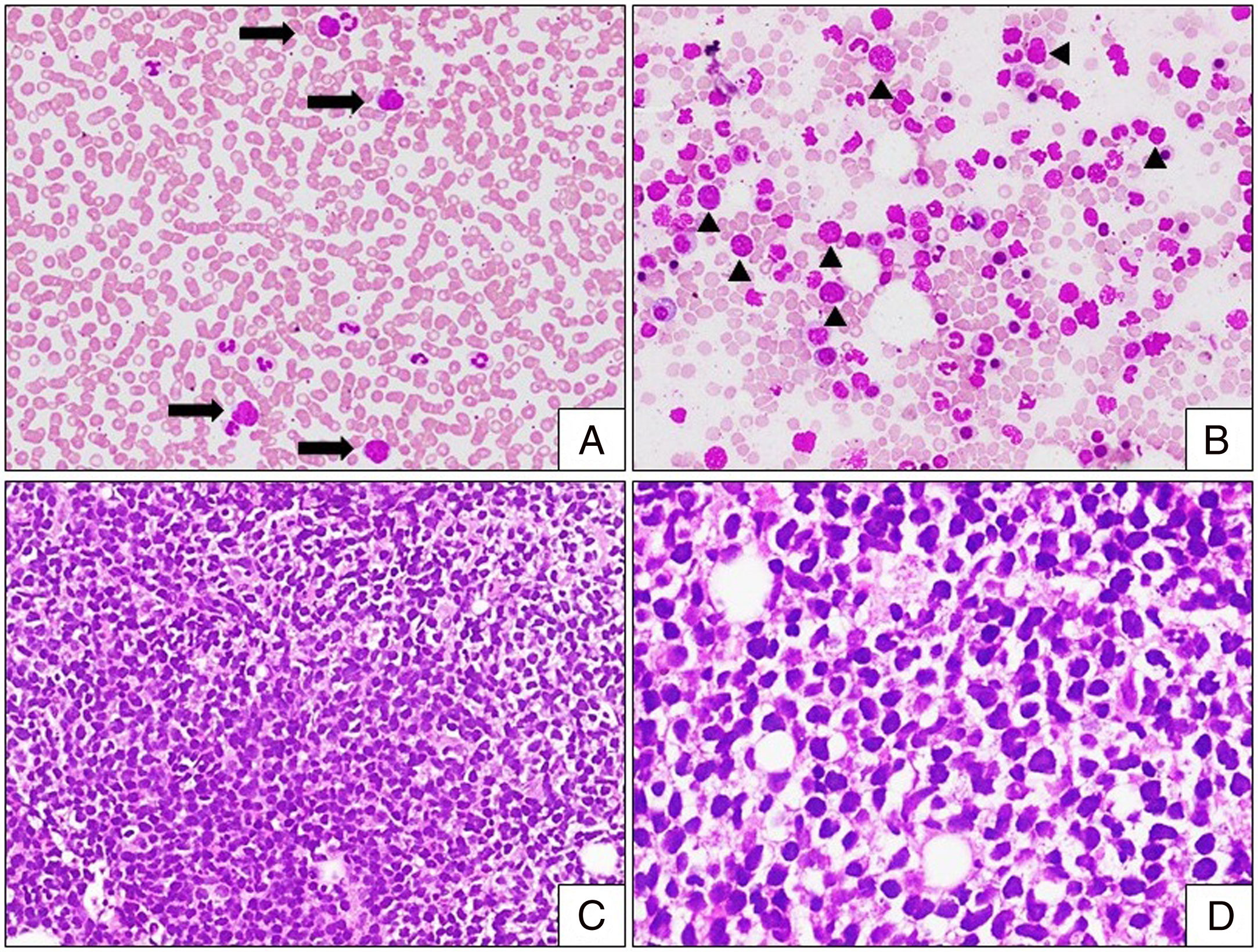
The hemogram revealed hemoglobin (13.4g/dl), and platelet count (206×109/L), with mild leucocytosis (19×109/L). The peripheral smear analysis revealed the presence of 5% of large pleomorphic abnormal lymphoid cells with a high nuclear-cytoplasmic (NC) ratio, sparse basophilic cytoplasm, enlarged irregular nuclei and irregularly clumped nuclear chromatin (Figure 2A). The bone marrow aspiration study revealed a hypercellular marrow, with the presence of similar large pleomorphic abnormal lymphoid cells (Figure 2B). Upon trephine biopsy, the marrow spaces were expanded and replaced by a monotonous population of abnormal medium-to-large-sized lymphoid cells, with a scant-to-moderate amount of cytoplasm, with a few showing cleaved nuclei (Figure 2C and D). Biochemical investigations revealed elevated serum lactate dehydrogenase levels (1053mg/dl) and normal creatinine (0.7mg/dl), uric acid (2.6mg/dl) and serum electrolytes.
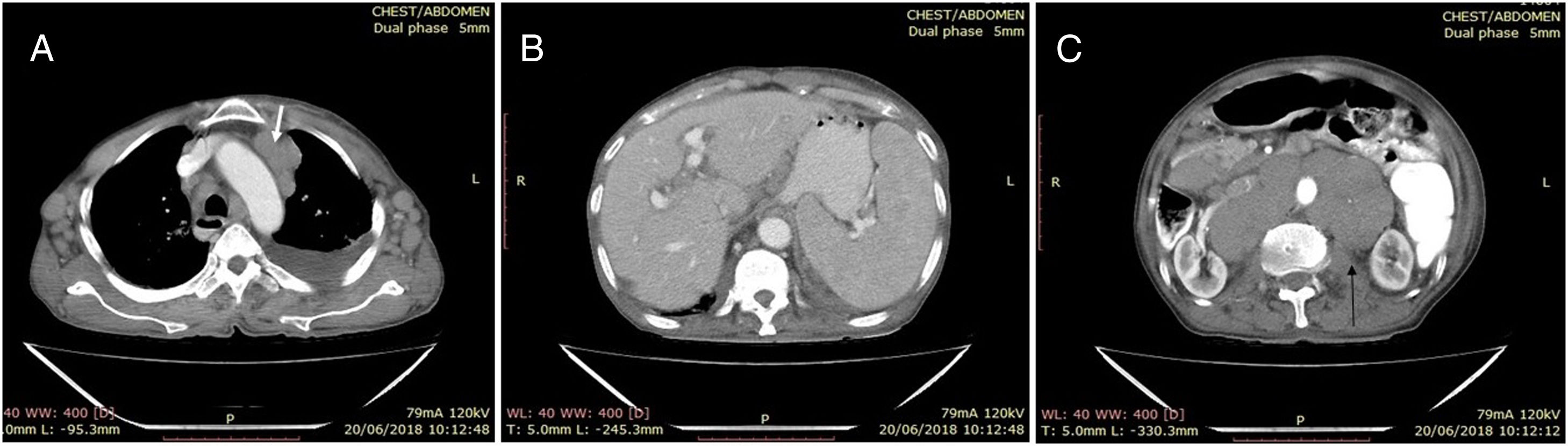
The neck ultrasound (USG) showed bilateral multiple enlarged hypoechoic cervical lymph nodes at the levels 1B, II, III and V. The largest lymph node was noted on the left level II, measuring 2.5cm×1.8cm. Thorax and abdomen contrast enhanced computed tomography (CECT) revealed bilateral mild pleural effusion (left>right), with subsegmental collapse of basal segments of the left lower lobe. Multiple, non-necrotic, bilateral homogenously enlarged lymph nodes in the upper and lower paratracheal, prevascular and axillary regions were observed (Figure 1A, white arrow), along with enlarged retroperitoneal lymph nodes (the largest measuring 3.6cm×2.7cm and located in the periportal region) and bilateral inguinal lymph nodes, the largest measuring 17mm×11mm, on the left side. The cerebrospinal fluid (CSF) examination was normal. Splenomegaly (Figure 1B) and multiple enlarged homogeneous lymph nodes in the retroperitoneum encasing the aorta (Figure 1C) were observed in the abdomen CECT examination.
Leukemic phase of DLBCL. (A) Peripheral smear with mild leukocytosis and presence of large abnormal lymphoid cells (arrow) [Leishman stain, 100×]. (B) Bone marrow aspiration with infiltration by abnormal lymphoid cells (arrowhead) with high NC ratio, some with nuclear clefts. Note the presence of bare nuclei in the background [Leishman stain, 100×]. (C and D) Bone marrow biopsy with marrow spaces completely replaced by sheets and vaguely nodular [(C); H&E stain, 200×] infiltrates of monotonous abnormal lymphoid cells. Note these cells exhibit moderate nuclear pleomorphism with nuclear membrane irregularity [(D); H&E stain, 400×].
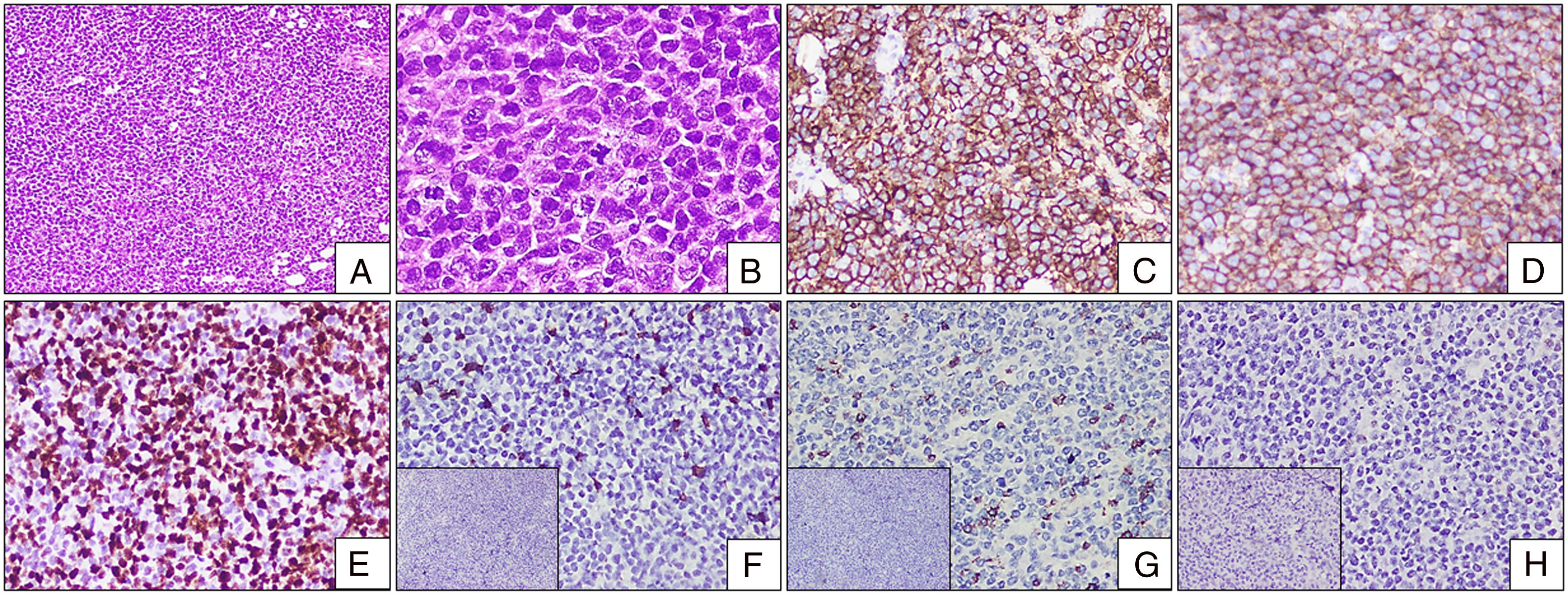
The histopathological examination of the cervical lymph node biopsy showed a complete effacement of the architecture and a replacement by a monotonous population of abnormal medium-to-large lymphoid cells. Scant cytoplasm with irregular round nuclei and clumped chromatin were striking (Figure 3A and B). Adjacent tiny lymph nodes showed necrosis surrounded by epithelial histiocytes, foamy histiocytes and giant cells. The stain for acid fast bacilli was negative. These lymphoid cells were diffuse positive for CD20 and CD10 and negative for CD3, transfusion dependent thalassemia (TDT), CD34, CD5, B-cell lymphoma 6 (BCL6) and multiple myeloma oncogene 1 (MUM1) in the immunohistochemistry (Figure 3C–H). B-lymphoblastic leukemia/lymphoma (B-LBL) was ruled out by the absence of CD34 and TDT expression in these lymphoid cells. The conclusion was that this case was the leukemic phase of diffuse large B-cell lymphoma, germinal center type. He was started on pre-phase chemotherapy and was scheduled for R CHOP chemotherapy, but he defaulted after the pre-phase.
Lymph node biopsy with IHC. (A and B) Lymph node biopsy with completely effaced architecture and diffusely infiltrated by sheets of monotonous abnormal lymphoid cells [(A) H&E stain, 100×]. The cells are pleomorphic with open chromatin and irregularly clumped chromatin. Note the presence of an atypical mitosis [(B) H&E stain, 400×]. (C) Diffuse bright membrane positivity for CD20 [H&E stain, 200×]. (D) Diffuse moderate membrane positivity for CD10 [H&E stain, 200×]. (E) High Ki-67 proliferative index with nuclear positivity [H&E stain, 200×]. (F) CD5 scattered positivity in background T cells. Inset: TDT negative [H&E stain, 200×]. (G) CD3 scattered positivity in background T cells. Inset: CD34 negative [H&E stain, 200×]. (H) BCL-6 negative [H&E stain, 200×]. Inset: MUM1 negative [H&E stain, 200×].
Lymphomas are usually detected at the primary site, such as the lymph node, as compared to chronic lymphocytic leukemia, which generally involves bone marrow. When there is an infiltration of lymphoma cells in the bone marrow and spillover into the peripheral blood, the leukemic phase of lymphoma arises, as observed in the present case. This leukemic phase is usually seen in its progressive phase or in stage IV disease and is extremely rarely found in DLBCL at the time of diagnosis. Only a few case reports or short series have been documented in the literature.1–5
The DLBCL is a complex disorder with varied clinical manifestations and histological patterns. It is known to have nodal as well as extra-nodal involvement. Extra-nodal involvement can be seen at sites, such as the gastrointestinal tract, central nervous system, thyroid, ovary, testis, etc. Histology shows that it is composed of large-to-medium-sized cells with moderate-to-relatively abundant cytoplasm, round-to-oval irregular nuclei, with coarsely clumped nuclear chromatin. Few nuclei show distinct-to-prominent nucleoli.1,2 The present case had similar morphological features.
Lymphomas are diagnosed principally based on the histological findings, but spillover of these lymphoma cells into the circulation (leukemic phase) can be diagnosed based on cellular immunophenotypic analysis by flow cytometry. Immunophenotypically, these cells show strong membrane positivity for B-cell lineage markers, such as CD19, CD20 and with follicular center markers, such as CD10 (40%) and BCl6 (60%). The non-germinal center type of DLBCL will show positivity for CD38 and MUM1.1,2 The BCl2 and CD5 expression in DLBCL is considered to represent a poor prognosis. They are known to have a high Medical Information Bureau (MIB) index.1,6,7 In the present case, the lymphoid cells show diffuse expression of CD20 and CD10 with high Ki-67 (more than 80%), favoring a Germinal Center type of DLBCL.
The B-cell NHLs, with intermediate and large abnormal lymphoid cells, are commonly encountered and have an aggressive clinical course with a poor prognosis if chemotherapy is not initiated. Many of these intermediate-to-large B-NHLs are categorized as DLBCLs and have variable therapeutic responses. However, other common hematologic malignancies need to be ruled out, such as B-LBL, mantle cell lymphoma, blastoid variant (MCL-Bv), and Burkitt lymphoma (BL).8 In the present case, B-LBL was ruled out with CD34 and TDT negativity, MCL-Bv, with CD5 negativity and BL, with BCL6 negativity.
Even though DLBCL is the most common NHL, the leukemic phase of the disease at presentation, as observed in the present case, is rarely reported. Other NHLs, such as mantle cell lymphoma, anaplastic large cell lymphoma, and follicular lymphoma, are known to have frequent peripheral blood dissemination.1 The reasons for the leukemic phase of DLBCL and its variable findings and clinical presentations are not clear. A possible mechanism for this could be the differential expression of adhesion molecules, but recent studies are still inconclusive. Patients in the leukemic phase of DLBCL are likely to have extra-nodal infiltration, not in the bone marrow, but rather in the spleen, lung, liver, bone, bowel, or CSF, thus increasing the disease burden.2
Rare cases are reported of DLBCL in the leukemic phase with multi-organ involvement and associated with the Epstein-Barr virus (EBV),9 The central nervous system (CNS) is involved with the BCL2 and Myc gene rearrangements10 and with the p53 gene rearrangement.11
A cohort study of 29 patients showed that about 90% of them were receiving combined anthracycline–rituximab-based regimens, associated with a higher early mortality of 14%, as compared to patients receiving the rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) regimen (6%). However, the 4-year survival rates of both regimens were comparable.2
ConclusionWe have described a rare case of leukemic presentation of DLBCL at diagnosis, whose systematic investigation led to an early diagnosis and initiation of treatment. The DLBCL in the leukemic phase should be considered as one of the differential diagnoses when there are intermediate-to-large abnormal lymphoid cells in the peripheral blood and bone marrow and the immunohistochemistry (IHC) should be considered to differentiate it from other B-cell hematologic malignancies.
Conflicts of interestThe authors declare no conflicts of interest.


![Leukemic phase of DLBCL. (A) Peripheral smear with mild leukocytosis and presence of large abnormal lymphoid cells (arrow) [Leishman stain, 100×]. (B) Bone marrow aspiration with infiltration by abnormal lymphoid cells (arrowhead) with high NC ratio, some with nuclear clefts. Note the presence of bare nuclei in the background [Leishman stain, 100×]. (C and D) Bone marrow biopsy with marrow spaces completely replaced by sheets and vaguely nodular [(C); H&E stain, 200×] infiltrates of monotonous abnormal lymphoid cells. Note these cells exhibit moderate nuclear pleomorphism with nuclear membrane irregularity [(D); H&E stain, 400×]. Leukemic phase of DLBCL. (A) Peripheral smear with mild leukocytosis and presence of large abnormal lymphoid cells (arrow) [Leishman stain, 100×]. (B) Bone marrow aspiration with infiltration by abnormal lymphoid cells (arrowhead) with high NC ratio, some with nuclear clefts. Note the presence of bare nuclei in the background [Leishman stain, 100×]. (C and D) Bone marrow biopsy with marrow spaces completely replaced by sheets and vaguely nodular [(C); H&E stain, 200×] infiltrates of monotonous abnormal lymphoid cells. Note these cells exhibit moderate nuclear pleomorphism with nuclear membrane irregularity [(D); H&E stain, 400×].](https://static.elsevier.es/multimedia/25311379/0000004200000002/v5_202107241647/S2531137919301002/v5_202107241647/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)
![Lymph node biopsy with IHC. (A and B) Lymph node biopsy with completely effaced architecture and diffusely infiltrated by sheets of monotonous abnormal lymphoid cells [(A) H&E stain, 100×]. The cells are pleomorphic with open chromatin and irregularly clumped chromatin. Note the presence of an atypical mitosis [(B) H&E stain, 400×]. (C) Diffuse bright membrane positivity for CD20 [H&E stain, 200×]. (D) Diffuse moderate membrane positivity for CD10 [H&E stain, 200×]. (E) High Ki-67 proliferative index with nuclear positivity [H&E stain, 200×]. (F) CD5 scattered positivity in background T cells. Inset: TDT negative [H&E stain, 200×]. (G) CD3 scattered positivity in background T cells. Inset: CD34 negative [H&E stain, 200×]. (H) BCL-6 negative [H&E stain, 200×]. Inset: MUM1 negative [H&E stain, 200×]. Lymph node biopsy with IHC. (A and B) Lymph node biopsy with completely effaced architecture and diffusely infiltrated by sheets of monotonous abnormal lymphoid cells [(A) H&E stain, 100×]. The cells are pleomorphic with open chromatin and irregularly clumped chromatin. Note the presence of an atypical mitosis [(B) H&E stain, 400×]. (C) Diffuse bright membrane positivity for CD20 [H&E stain, 200×]. (D) Diffuse moderate membrane positivity for CD10 [H&E stain, 200×]. (E) High Ki-67 proliferative index with nuclear positivity [H&E stain, 200×]. (F) CD5 scattered positivity in background T cells. Inset: TDT negative [H&E stain, 200×]. (G) CD3 scattered positivity in background T cells. Inset: CD34 negative [H&E stain, 200×]. (H) BCL-6 negative [H&E stain, 200×]. Inset: MUM1 negative [H&E stain, 200×].](https://static.elsevier.es/multimedia/25311379/0000004200000002/v5_202107241647/S2531137919301002/v5_202107241647/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)



