Chronic lymphocytic leukemia is the most common hematologic malignancy among adults in Western countries. Several studies show that somatic mutations in the TP53 gene are present in up to 50% of patients with relapsed or refractory chronic lymphocytic leukemia. This study aims to review and compare the methods used to detect somatic TP53 mutations and/or 17p deletions and analyze their importance in the chronic lymphocytic leukemia diagnosis and follow-up. In chronic lymphocytic leukemia patients with refractory or recurrent disease, the probability of clonal expansion of cells with the TP53 mutation and/or 17p deletion is very high. The studies assessed showed several methodologies able to detect these changes. For the 17p deletion, the chromosome G-banding (karyotype) and interphase fluorescence in situ hybridization are the most sensitive. For somatic mutations involving the TP53 gene, moderate or high-coverage read next-generation sequencing and Sanger sequencing are the most recommended ones. The TP53 gene mutations represent a strong adverse prognostic factor for patient survival and treatment resistance in chronic lymphocytic leukemia. Patients carrying low-proportion TP53 mutation (less than 20–25% of all alleles) remain a challenge to these tests. Thus, for any of the methods employed, it is essential that the laboratory conduct its analytical validation, documenting its accuracy, precision and sensitivity/limit of detection.
Chronic lymphocytic leukemia (CLL) is the most frequent hematologic malignancy among adults in Western countries. It affects mainly the elderly population, being rare in individuals aged <40 years. Most patients live 5–10 years from diagnosis, while some die earlier due to complications from the disease.1,2
CLL is characterized by a progressive accumulation of CD5+ B-cell lymphocytes in the peripheral blood, lymph nodes, spleen and bone marrow. The disease is extremely heterogeneous from both a biological and a clinical point of view. The diagnosis is based mainly on the complete blood count (CBC) and the morphological evaluation of a blood smear, which show leukocytosis with lymphocytosis (>5000/µL) and on immunophenotyping of the peripheral blood circulating B-cells, which identifies a clonal B-cell population with the CD5 marker, in addition to B-cell markers (CD19, CD20, CD22 and CD23).3,4 Of note, the Brazilian Group of Flow Cytometry (GBCFLUX) consensus provides important recommendations for the diagnosis of CLL.4–6 The risk is higher among men, with a ratio of 1.7 men affected for each woman with the disease.7 The incidence of CLL in the US between 2006 and 2010 was 4.2 cases per 100,000 individuals, considering all ages.8 Among individuals aged <65 years, the incidence was 1.3/100,000, increasing to 24.8/100,000 in the population aged >65 years.8 In Brazil, there are no estimates on the specific incidence of CLL. Considering all leukemias combined, the estimate for 2016 is 4.4 cases per 100,000 women and 5.6 cases per 100,000 men.9
The CLL treatment has substantially evolved in recent decades. The median survival increased from 5 to 7 years in the 70 s to 10–12 years in the present day.10 In a recent publication by the Brazilian Registry of CLL, which included data from 1903 patients, the overall survival was 88% at 3 years and 82% at 5 years.11 One of the pillars in this evolution was the identification of molecular changes associated with response to therapy. These changes are the main prognostic factors of overall survival.10,12
Mutations in the immunoglobulin heavy chain variable region (IGHV) genes and mutations and/or deletions in the TP53 gene, mapped in 17p, are among the most relevant molecular somatic changes in CLL research and treatment. Recently, an international index for prognosis of the disease was developed, the Chronic Lymphocytic Leukemia - International Prognostic Index (CLL-IPI), which includes these genetic alterations, serum β2-microglobulin levels, patient age and clinical stage according to the Rai and Binet classifications.13
Del 17p/TP53 alterations are the most important prognostic and predictive markers for treatment decisions in CLL.14 They have been shown to convey resistance to standard chemo(immuno)therapies, such as fludarabine, cyclophosphamide and rituximab. In a clinical trial (CLL4) that compared first-line treatment with chlorambucil versus fludarabine, with or without cyclophosphamide, progression-free survival (PFS) and overall survival (OS) were significantly shorter in TP53mut patients than in patients with wild-type TP53, independent of the allocation group.15 In the CLL8 trial, that evaluated first-line therapy with fludarabine and cyclophosphamide (FC) or FC with rituximab (FCR) among patients with untreated CLL, TP53 alterations also had a significant negative impact on both the OS and PFS.16 Similarly, the complex karyotype predicted the lack of response to chlorambucil and rituximab as first-line induction treatment, with or without rituximab as maintenance, in a study with elderly CLL patients.17 Until a few years ago, alemtuzumab and allogeneic stem cell transplantation were the only effective treatments for those patients. Recently, ibrutinib, idelalisib and venetoclax, which inhibits the Bruton tyrosine kinase (BTK), the phosphatidylinositol 3-kinase (PI3K) and BCL2, respectively, have been approved, based on their efficacy in CLL clinical trials with patients harboring del17p/TP53 mutations.10
The p53 protein is a crucial transcription factor for the cell because it controls several biological processes, such as cell cycle, differentiation, DNA repair, apoptosis and angiogenesis.18 Defects in the p53 pathway prevent DNA repair and apoptosis, resulting in genomic instability and abnormal cell proliferation.18 Several studies analyzing sequential samples from CLL patients documented the apparent acquisition of 17p deletions and TP53 mutations and the expansion of the cell population with these changes (clonal expansion). According to these studies, abnormalities in TP53 are present in up to 50% of the patients with relapsed or refractory disease and only 5–15% of newly diagnosed cases or immediately before the first treatment.15,16,19–22
In 2015, Landau et al. published a study that observed a concordance between the increase of del(17p) and small mutations in TP53 after treatment and relapse in all 12 cases of CLL presenting the two types of event; furthermore, no new TP53 mutation was detected in any of them over time. Based on these and other results and a paper published in the journal Cell in 2013 by Davoli et al. the authors suggested that the TP53 gene is one of the few tumor suppressors in which the inactivation of the two alleles (the famous two-hit hypothesis proposed by Knudson) is really necessary for clonal expansion.23 Similar results were published by Amin et al. in 2016.24
Recent studies, based on high sensitivity methods, have detected TP53 mutations in subclones. These studies have shown that the proportion of CLL cases with TP53 mutations at diagnosis/treatment initiation is much higher than previously thought and that, even at the subclone level, such mutations may confer resistance to clonal treatment and expansion.25–27 Based on these findings, most medical societies, including the Brazilian Group of CLL, recommend the investigation of 17p deletions and TP53 mutations before initiating first-line treatment or any further lines.4
The objective of this study was to conduct a review of the tests to detect somatic TP53 mutations and 17p deletions and thereafter analyze their importance for the diagnosis and long-term follow-up of CLL.
Tests for detection of 17p deletionsFISH and karyotypeThe FISH is a sensitive and quantitative method for the detection of del(17p) and is the method of choice of most laboratories to date. It is well standardized and reproducible.28 Importantly, the cut-off value for the percentage of del(17p) cells that predict poorer outcomes, regarding overall survival and progression-free survival, has been established as 10–20%, a value that is undoubtedly within the FISH limit of detection.29–31
The FISH analysis is recommended before the initiation of therapy for progressive CLL and is mandatory in cases of chemotherapy with alkylating agents or purine analogues. Given the risk of clonal expansion in CLL patients, the FISH should be repeated at least before each new treatment in all patients with no deletion detected in a previous sample, especially in those with treatment-refractory CLL or who show an aggressive clinical course.4,32,33
One limitation of the FISH is that it cannot detect the so-called complex karyotype, defined as the presence of multiple cytogenetic, numerical and/or structural changes. Conventional cytogenetics (karyotyping) can detect those alterations and should be performed, therefore, along with the FISH. The complex karyotype is also associated with a poor prognosis in CLL.34 In a recent paper, Herling et al.35 analyzed 161 CLL patients by chromosome G-banding in stimulated cultures, FISH and NGS for a panel of 85 genes from peripheral blood samples. The karyotype analysis revealed that 69% of the patients presented chromosomal alterations, 31%, translocations and 20%, the complex karyotype. They also found that 76% of the patients had mutations in one of the panel genes, including the TP53. A particularly noteworthy finding was that the complex karyotype and changes involving TP53 (mutations or deletions) acted as independent prognostic factors.
Other methodsOther methods that can be used to detect del(17p) and other deletions and insertions are the single-nucleotide polymorphism (SNP) array, comparative genomic hybridization (CGH) array and multiplex ligation-dependent probe amplification (MLPA). Of note, studies performed to date suggest that these methods have lower sensitivity, when compared with the FISH, failing to detect clones smaller than 20–30% of the total cells.32,36
A promising method to identify subclones that correspond to at least 10% of the total B cells is the NGS. Although current CLL treatment algorithms still do not incorporate recommendations for the detection of subclones, researches have shown that subclonal TP53 mutations and the del17p are associated with overall survival rates as short as those observed in CLL patients with clonal defects.27,37 As the detection of large deletions and duplications has been the object of optimization in several studies on the NGS, eventually this should also become the methodology of choice for the detection of the del(17p).38–40
Tests for detection of somatic TP53 mutationsBased on its predictive value, all current treatment guidelines for CLL recommend prior testing for TP53 mutations.41–43 The testing should be repeated with each new disease progression demanding treatment because of the expansion of altered clones.37,42
Specific methods and conditions for detecting small mutations in the TP53 vary significantly between laboratories. To reduce this variability, the European Research Initiative on CLL (ERIC),44 published in 2012, indicated the most appropriate methods for detecting TP53 mutations and the criteria for the analysis42: Sanger sequencing, denaturing high-performance liquid chromatography (DHPLC), functional analysis of separated alleles in yeast (FASAY), arrays and NGS. Given the increased sensitivity of the NGS approach, the ERIC has recently updated its recommendations for the TP53 mutation analysis in CLL, but has also advised that pre-screening methods, such as the DHPLC, are still cost-effective.45
For cases in which the proportion of leukemic cells in peripheral blood is less than 60%, the ERIC recommended the use of bone marrow, lymph nodes or CD19+ cell selection before the test, especially for the Sanger sequencing analysis, whose sensitivity is usually lower than the other methods. The guidelines also reinforced that, for most methods considered, a new PCR reaction followed by Sanger sequencing should be conducted to confirm the mutations.42,45
ArraysTwo array platforms are most commonly used: GeneChip p53 from Affymetrix or AmpliChip p53 from Roche.46 Both are based on DNA hybridization with oligonucleotide probes immobilized on a chip and a platform based on the extension of primers connected to the array, using the tested DNA as a template. In the first case, each mutated base is represented by at least two probes, wild and mutated. The DNA is marked with fluorescence before the hybridization and the signal intensity of each of the probes is checked for the analysis of the result. The method based on the extension of primers connected to the array has as a principle the incorporation of one of four dideoxynucleotides marked with different colors (fluorophores). The primer attached to the array is a base sequence of the TP53 gene that terminates just before the queried (potentially mutated) base. The PCR products obtained from the DNA are hybridized with the array and the base added to the immobilized primer is complementary to the DNA.47
The AmpliChip, resulted from the development of the GeneChip, which was the first generation of chips. It was designed to detect all single-base substitutions and deletions of a single nucleotide in exons 2–11 of the TP53 gene at the respective splice sites and to achieve a detection limit of 25% of mutated alleles.42,46 The primer extension array was designed to detect 95% of the known mutations in exons 2–9 of the TP53 gene.42,47 The main advantages are the speed and practicality of the tests. Moreover, they do not require extensive optimization. In addition to the cost (of equipment and reagents), a significant drawback of this method is the detected mutation spectrum, which only includes those whose probes have been bound to the array and substitutions or deletions that include only one nucleotide.42
In the follow-up study on the ERIC recommendations, the AmpliChip detected 71% of the TP53 mutations and 81% of the patients with mutations. Considering the detection limit and the types of mutations potentially detectable, the AmpliChip identified 91% of the mutations in 91% of the patients. Furthermore, among the 45 mutations present in less than 25% of the alleles, 25 (56%) were detected. The spectrum of mutations identified by this method was the same as that of the DHPLC analysis followed by the Sanger sequencing.48
Dicker et al.34 also used the DHPLC followed by the Sanger sequencing and AmpliChip p53 to screen TP53 mutations in 193 CLL patients. By the DHPLC/Sanger sequencing, 24 of the 33 (73%) mutations were detected in 20 (77%) patients. Thus, the DHPLC/ Sanger sequencing detected 75% of the mutations in 80% of the patients. The AmpliChip detected 30 mutations (91%) in 25 patients (96%). The AmpliChip did not detect three indels. On the other hand, it detected eight mutations that were not confirmed by sequencing. The discrepancy between the two methodologies was a consequence of the low sensitivity of the sequencing (10% of the alleles), since the DHPLC initially detected the eight mutations.
Sanger sequencingFor the Sanger sequencing, it is preferable to use genomic DNA than cDNA because some mutations in the TP53 may lead to mRNA degradation (decay of nonsense mRNA). Exons 4–10 must be analyzed, as they account for more than 95% of the mutations.42,45
Although it is a method available in most laboratories/centers and is considered the gold standard in the precise identification of the mutation, the Sanger sequencing is relatively expensive and time-consuming. Furthermore, this method may fail to detect subclonal mutations when the mutated allele accounts for less than 20–25% of all the alleles.42 These small mutated subclones are present in a significant percentage of CLL patients and might imply a prognosis as poor as that associated with more expanded clones harboring TP53 mutations.23,26,27
Next-generation sequencing (NGS)Given its sensitivity and throughput, the targeted NGS is considered the gold-standard method for detecting somatic mutations in the TP53 gene.23,25,26,35,49–51 It also takes less time than other methods when it is necessary to analyze a large number of samples or many regions of the genome. Several commercial kits are currently available for the preparation of the sequencing reactions of the TP53 and other disease-related genes (such as relevant gene panels in oncology), either separately or in a multiplex format. Depending on the purpose, NGS platforms can be used to examine a large number of samples simultaneously or only a few samples for various genomic and/or deep sequencing regions.42 The deep or ultra-deep sequencing allows detecting mutations with very low representability (subclones) at very high sensitivity.
In the follow-up study on the ERIC recommendations published in 2012, samples from 69 patients were tested by ultra-deep NGS, with a median coverage of 27,538 reads per base (range 2096 to 88,976 reads per base). The ultra-deep NGS identified all 58 mutations in the TP53, also detected in 37 patients by at least one of the other methods (FASAY, DHPLC and AmpliChip). In addition, other subclonal mutations were identified in 24 (65%) of these patients. Among 32 patients classified as non-mutated by other methods, the NGS detected subclonal mutations (represented by 0.2%–3.8% of the alleles) in eight (25%) patients. In five of them, the FASAY detected the expansion of each mutation in a subsequent sample.48 Regarding the correlation between the Sanger sequencing and NGS, a number of studies showed a higher sensitivity of the latter; while all variants detected by the Sanger sequencing are also detectable by the NGS, the opposite is not true.27,45,48,52,53
The primary challenge in using the NGS is data analysis, particularly in the distinction between subclonal mutations and artifacts; thus, it is essential to have a bioinformatics professional as a team member. To detect mutated alleles present at low levels, the coverage (number of reads per base) must be high and the error introduced during the procedure (polymerase errors, for example) must be low. Thus, the use of a polymerase with high fidelity (proofread) significantly improves the detection limit of the method. Statistical analysis tools for results are under development.42
The deep sequencing methodology was used in several recent studies that demonstrated the importance of subclonal mutations in the TP53 as a predictive factor in CLL. These studies verified that the resistance to treatment is associated with the expansion of these subclones, already present at the beginning of treatment, but undetectable by other methods, and that the acquisition of new abnormalities in the TP53 in untreated patients is a rare event.25,27,54
In one of these studies, the ultra-deep NGS evaluated 309 newly diagnosed CLL patients and detected 85 TP53 mutations in 46 (15%) of them, including mutations accounting for only 0.3% of the alleles. The Sanger sequencing did not detect 50 of the 85 mutations (59%) in 15 patients. Patients with subclonal mutations had the same clinical phenotype and survival (low) as patients with clonal mutations. The study analyzed subsequent samples and found that the small subclones identified before the treatment became the predominant cell population in relapse and anticipated the development of refractoriness to therapy.27
In another study, the authors re-analyzed samples from 20 patients initially classified as non-mutated by the FASAY and who had a TP53 mutation detected in relapse, as well as samples from 40 patients who remained unchanged after relapse. They used the deep NGS and a high-fidelity polymerase to minimize sequencing errors. Among the 20 relapsed patients, subclonal mutations were detected in the TP53 (in 0.20–3.71 % of the reads) in 18 (90%) of them. Among the 40 patients without recurrence, only one presented a mutation in 0.55% of the reads. Analyzing subsequent samples from this patient, it was verified that there was no expansion of the clone.25
One important limitation of the NGS method is its high cost and the fact that those pieces of equipment are not available at most medical centers, particularly in poor countries.
Other methodsOlder methods based on gel electrophoresis are still used by some laboratories. Examples of these methods are the SSCP (single strand conformation polymorphism) and the DGGE (denaturant gradient gel electrophoresis), which can detect approximately 5% of the mutated alleles. They do not demand expensive equipment, but are relatively laborious (especially the latter) and need extensive optimization. Also, they do not identify the precise mutation, so they are considered as initial screening methods (such as the FASAY and DHPLC), with a subsequent need for altered samples.18
Methods for the enrichment of the mutated allelePurification of lymphocytes or CD19+ cells from the peripheral blood promotes the enrichment of the mutated alleles in the sample and is used in most of the studies and tests in CLL. However, considering the need to identify mutations at very low subclonal levels (<1%), greater enrichment would have to be performed before most of the methods mentioned above, except the deep NGS. The COLD-PCR (coagulation at a lower denaturation temperature - PCR)55 and the DISSECT (differential strand separation at a critical temperature)56 are methods that allow the enrichment of the mutations by approximately 100–200 times. The former is based on preferential denaturation of the mutated DNA during PCR reactions in which the denaturation temperature is reduced. Several denaturation temperatures varying by 0.3 °C are used during the reaction to allow enrichment of different types of mutations. Thus, the mutated sequences are preferably amplified, regardless of their location in the amplicon.55 As the interaction between primers with non-specific product formation is usually higher in the COLD-PCR, compared to the conventional PCR, it is recommended to conduct the PCR using water-in-oil emulsion.57
The DISSECT is based on repeated cycles of hybridization and lower temperature denaturation between the targeted DNA and probes representing wild segments of the region/gene to be analyzed. The probes are attached to a solid support (magnetic beads). As the denaturation temperature is reduced, the DNA that will dissociate from the probe will preferably be the mutated one and it will be eluted in the supernatant. Most of the wild-type DNA will remain bound to the beads, which are removed from the solution with the help of a magnet.56
It is important to note that these methods enrich only mutations that promote a reduction in the melting temperature of the amplicon. Furthermore, both require a pre-amplification step of the total genome.55,56
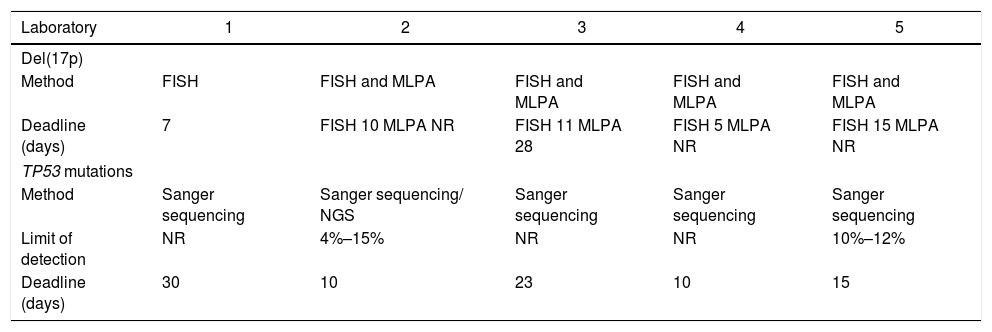
TP53 mutation detection in BrazilAll five laboratories that answered the survey regarding the TP53 mutation detection in Brazil rely on the Sanger sequencing (4 laboratories) or the NGS and Sanger sequencing (1 laboratory) (Table 1). Only two laboratories reported the limit of detection of their methods and the one that uses the NGS and Sanger sequencing reported a slightly greater sensitivity than the one that relies only on the Sanger.
Tests for del(17p) and TP53 mutations in some of the main laboratories in Brazil.
| Laboratory | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Del(17p) | |||||
| Method | FISH | FISH and MLPA | FISH and MLPA | FISH and MLPA | FISH and MLPA |
| Deadline (days) | 7 | FISH 10 MLPA NR | FISH 11 MLPA 28 | FISH 5 MLPA NR | FISH 15 MLPA NR |
| TP53 mutations | |||||
| Method | Sanger sequencing | Sanger sequencing/ NGS | Sanger sequencing | Sanger sequencing | Sanger sequencing |
| Limit of detection | NR | 4%–15% | NR | NR | 10%–12% |
| Deadline (days) | 30 | 10 | 23 | 10 | 15 |
FISH, fluorescence in situ hybridization; MLPA, multiplex ligation-dependent probe amplification; NR, not reported; NGS, next-generation sequencing.
In patients with CLL who have undergone at least one treatment line (i.e., who represent refractory or recurrent cases), the probability of the clonal expansion of cells with the TP53 mutation and/or 17p deletion is very high. Thus, several methodologies will be able to identify such changes. To detect the 17p deletion, the chromosome G-banding (karyotype), FISH and arrays (SNP-array or CGH-array) or moderate or high-coverage read NGS (about 100 or more reads per base) are considered the most effective ones. Also, to identify other somatic mutations involving the TP53 gene, the Sanger sequencing, preceded or not by a screening method (ideally DHPLC) or a moderate or high-coverage read NGS (about 100 or more reads per base), is more indicated.
In newly-diagnosed CLL patients or in those who have not yet undergone treatment, the sensitivity of some of these methods implies in false-negative results in cases with a low proportion of mutated cells (<10–20% for small TP53 mutations and <5% for 17p deletions). For these patients, the ideal method would be to use the high-coverage/deep sequencing NGS (about 1000 or more reads per base) and high-fidelity polymerase. In the case of small mutations, the Sanger sequencing preceded by a method of enrichment of mutated alleles, such as the COLD-PCR or DISSECT, could also be suitable (also using a high-fidelity polymerase).
For any of these methods, it is essential that the laboratory perform analytical validation, documenting its accuracy, precision and sensitivity/detection limit. In Brazil, there are at least five clinical laboratories that offer the FISH test to detect 17p deletions and the Sanger sequencing for TP53 mutations. The NGS is available in at least one of those labs.
Of note, the detection of the del(17p) and TP53 mutations plays a crucial role, regarding the treatment definition for CLL patients. Cases harboring those alterations do not benefit from the FCR scheme and other chemoimmunotherapy combinations that have resulted in the dramatic improvement in the response and survival rates of most CLL patients in the last decades.58–60 Treatment options for patients with the del(17p) and/or TP53 aberrant clones include ibrutinib, venetoclax, alemtuzumab, obinutuzumab, high-dose methylprednisolone (HDMP) and rituximab, among others. Stem cell transplantation in also an option in healthy patients who show disease progression.60,61
Financial supportThis study was sponsored by Abbvie.
Conflicts of interestThis study was sponsored by Abbvie.
AbbVie participated in the interpretation of data, review and approval of the content. All the authors had access to all relevant data and participated in writing, review and approval of this manuscript. Eloisa Moreira from Kantar provided data collection, data analysis, literature review and medical writing services in the development of this manuscript, funded by AbbVie.