Cytomegalovirus infection and disease are significant causes of morbidity and mortality among patients with hematopoietic stem cell transplantation. The aim of this study was to assess the frequency of cytomegalovirus infection and characterize the patients who developed the disease.
MethodsA retrospective cohort study was performed among adult patients, recipients of allogeneic HSTC between 2008 and 2015. Taking into account the institutional protocol of prophylaxis infections in hematopoietic stem cell transplantation, patients received either preemptive therapy or prophylaxis with valganciclovir. Infection was defined as a positive pp65 antigenemia assay or PCR higher than 500copies/mL. Disease was defined as viremia with evidence of end organ damage.
ResultsSeventy patients were included, the median age was 36 years old (IQR 17–62). A total of 93% of the recipients had a positive serology. The Cytomegalovirus infection occurred in 59% of the patients. Eleven patients developed disease (16%), the most frequent manifestation being colitis, followed by pneumonitis and a single case of retinitis. There were no differences between the preemptive therapy or prophylaxis groups. The mean time of onset of the disease was day 94 post-transplant. Three patients developed disease with a viral load lower than 1000copies/mL.
ConclusionThe incidence of cytomegalovirus infection after transplantation at our institution is high. It was found that the disease can occur with any level of viral load and is associated with high mortality.
Since 1957, when E. Donall Thomas reported the first allogeneic hematopoietic stem cell transplantation, there have been numerous advances in technique, donor selection, detection and treatment of complications. These advances have been reflected in a significant reduction of transplant-related mortality over the years.1
In spite of multiple strategies to prevent post-transplant infections, cytomegalovirus (CMV) continues to be a catastrophic issue among this population. Infection by CMV usually occurs in the first 100 days after transplantation, associated with an immunosuppression status. The infection can evolve and cause disease in the gastrointestinal tract, central nervous system, lungs and retina; this end organ damage is potentially fatal in these patients.2
Many studies have reported the incidence and risk factors associated with CMV disease. Nevertheless, the impact that this entity has on the population in our setting is unknown.
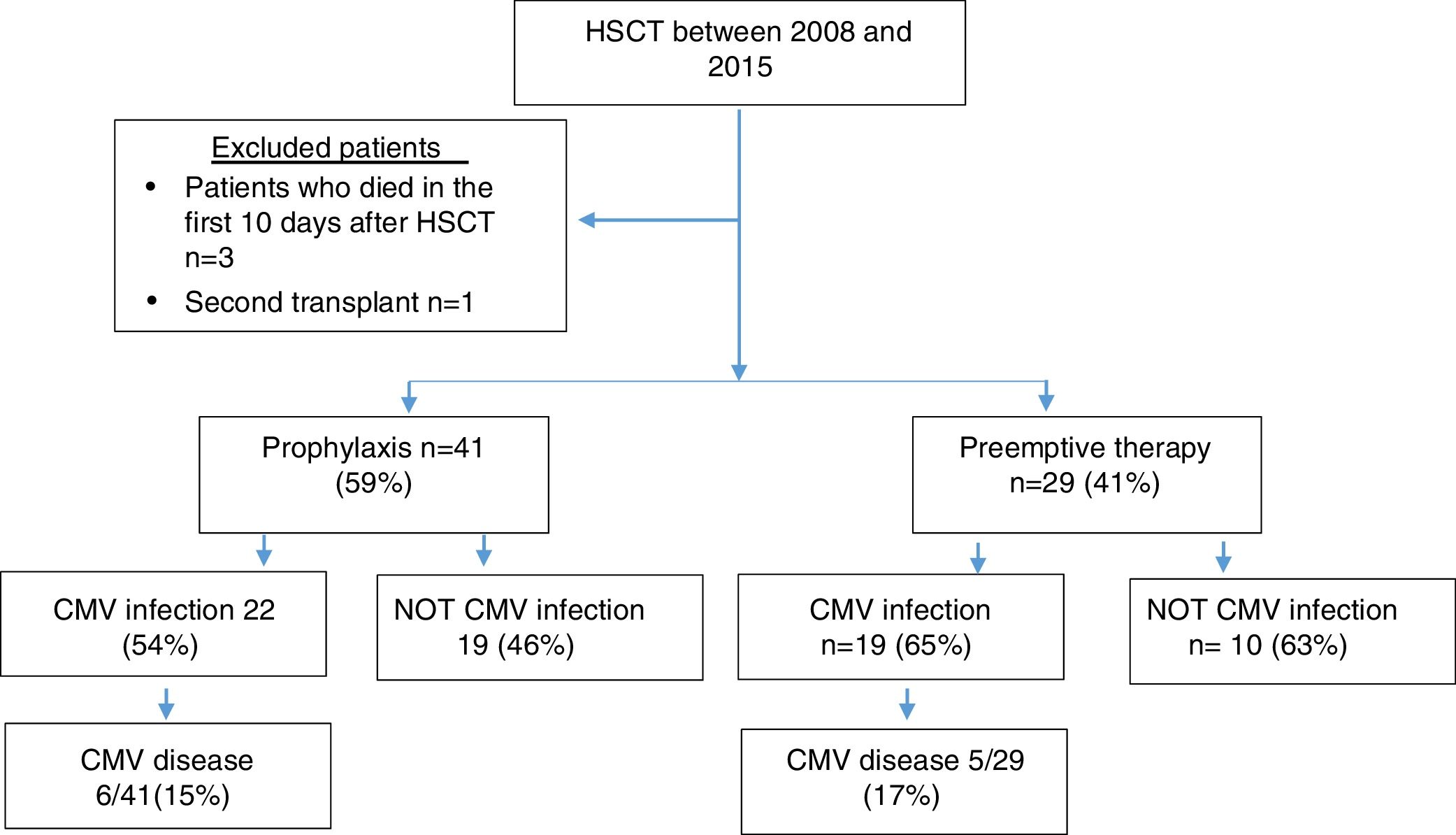
MethodsPatientsPatients older than 18 years with a diagnosis of benign or malignant hematological pathology who underwent allogeneic hematopoietic stem cell transplantation (HSCT) between 2008 and 2015 at Fundacion Valle del Lili, Cali-Colombia were included in this study. Those patients who died in the first 10 days after transplantation and patients undergoing a second transplant were excluded from the study.
Conditioning regimenConditioning regimens varied according to the indication for transplantation and the donor type. All regimens were myeloablative. Patients diagnosed with aplastic anemia received anti-thymocyte globulin of equine origin. Haploidentical transplants were performed with post-transplant cyclophosphamide for the prevention of graft-versus-host disease (GVHD).
The pre-transplant risk was assessed according to the European Group for Blood and Marrow Transplantation (EBMT) risk score.3
GVHD prophylaxisMost patients received GVHD prophylaxis with cyclosporine combined with methotrexate, cyclophosphamide or steroids. Diagnosis and grading of GVHD was assessed through clinical and laboratory findings according to the Glucksberg scale criteria.4
Definitions of infection and diseaseThe diagnosis of CMV infection was made by the detection of a viral load >500copies/mL or positive pp65 antigenemia in two consecutive assays; the diagnosis of CMV disease was established upon the presence of symptoms, clinical signs or manifestations of end-organ damage reflected in laboratory tests and confirmed by biobsy.5
Prophylaxis, follow-up and treatment of CMVPrevention strategies for CMV infection were based either on prophylactic or preemptive approaches. Patients who received prophylaxis were administered valganciclovir 900mg every 12h, once the myeloid graft was established up to 3 months post-HSCT. Preemptive therapy consisted in weekly monitoring for CMV after myeloid graft using the pp65 antigen between 2008 and 2011 or quantitative polymerase chain reaction (PCR) since 2012. The tests were performed weekly during the first 3 months, then every 2 weeks or upon each control follow-up until the first year post-HSCT.
When viremia was detected, either by a PCR >500copies/mL or positive pp65, patients received induction therapy with valganciclovir 900mg orally every 12h, ganciclovir 5mg/kg intravenously every 12h or Foscarnet 60mg/kg intravenously every 8h. This was followed by consolidation therapy with valganciclovir 900mg orally every 24h, ganciclovir 5mg/kg intravenously every 24h or Foscarnet 90–120mg/kg intravenously every 24h, until achieving two consecutive negative viral loads.
The treatment of the disease consisted in administering ganciclovir 5mg/kg every 12h, valganciclovir 900mg every 12h or foscarnet in the cases in which the patient presented severe aplasia, at a dose of 90mg/kg/12h for 2 weeks, followed with 120mg/kg/day.
Statistical analysisA descriptive statistical analysis was performed for all the selected variables and subgroups. The categorical variables are presented in proportions, the continuous variables are expressed as median with their interquartile range (RIC) or averages±standard deviation (SD). The primary outcomes of the study were: viremia, cytomegalovirus disease and death. The survival analysis was performed with the Kaplan–Meier method, and for the comparisons between subgroups the Log-rank test was applied. A risk analysis was carried out according to the type of transplant, prevention behavior and the presence of CMV disease using the Haenszel tablecloth test. The statistical package Stata (STATA) 12 version was used to analyze the data.
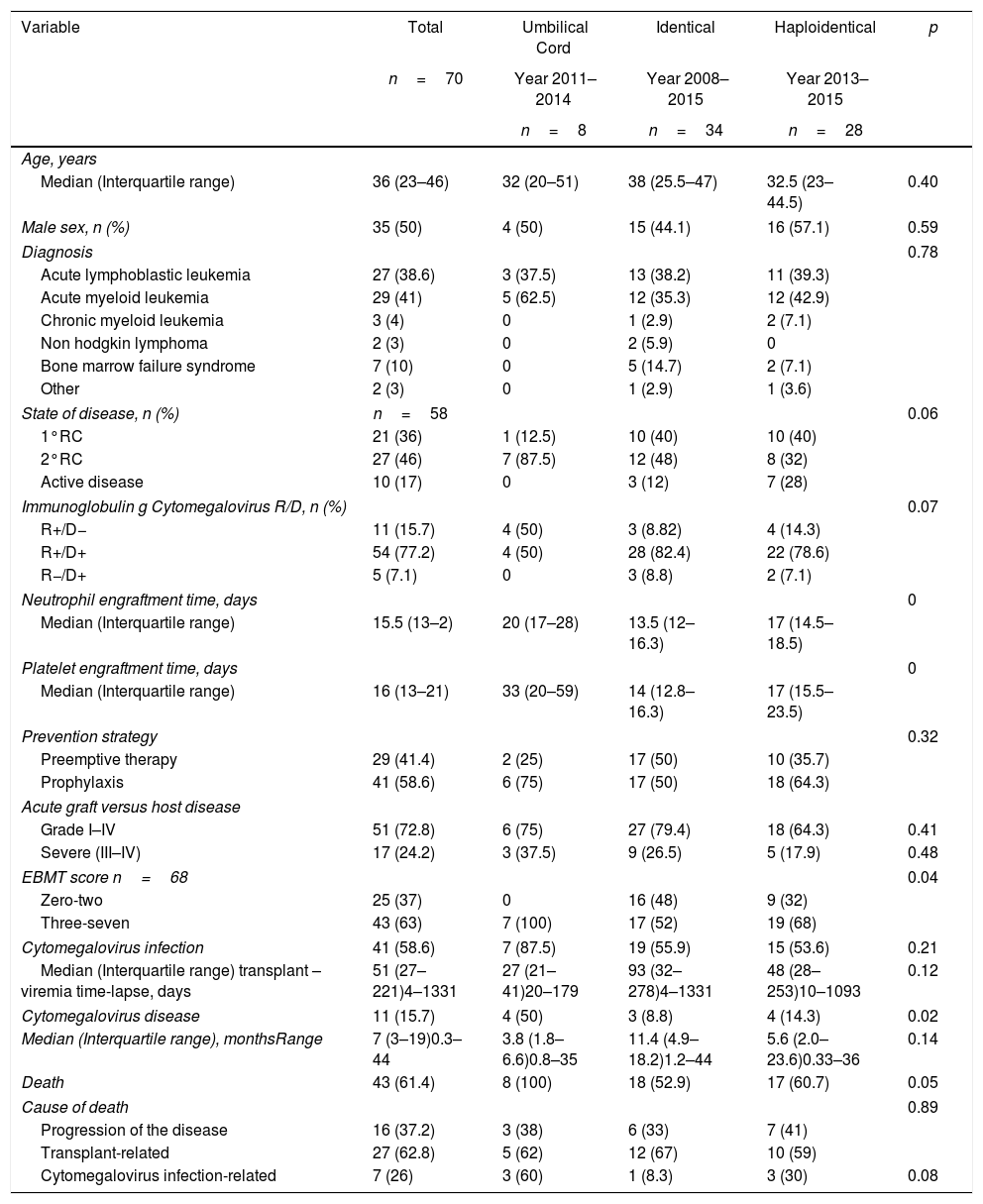
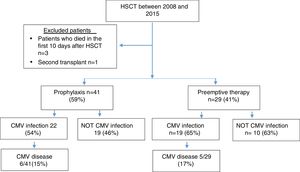
ResultsSeventy patients were included in the study (Figure 1), and the clinical characteristics according to the type of transplant performed are presented in Table 1. The median age at the time of transplant was 36 years, and most of the transplants were performed in patients with a diagnosis of acute leukemia.
Clinical characterization of patients.
| Variable | Total | Umbilical Cord | Identical | Haploidentical | p |
|---|---|---|---|---|---|
| n=70 | Year 2011–2014 | Year 2008–2015 | Year 2013–2015 | ||
| n=8 | n=34 | n=28 | |||
| Age, years | |||||
| Median (Interquartile range) | 36 (23–46) | 32 (20–51) | 38 (25.5–47) | 32.5 (23–44.5) | 0.40 |
| Male sex, n (%) | 35 (50) | 4 (50) | 15 (44.1) | 16 (57.1) | 0.59 |
| Diagnosis | 0.78 | ||||
| Acute lymphoblastic leukemia | 27 (38.6) | 3 (37.5) | 13 (38.2) | 11 (39.3) | |
| Acute myeloid leukemia | 29 (41) | 5 (62.5) | 12 (35.3) | 12 (42.9) | |
| Chronic myeloid leukemia | 3 (4) | 0 | 1 (2.9) | 2 (7.1) | |
| Non hodgkin lymphoma | 2 (3) | 0 | 2 (5.9) | 0 | |
| Bone marrow failure syndrome | 7 (10) | 0 | 5 (14.7) | 2 (7.1) | |
| Other | 2 (3) | 0 | 1 (2.9) | 1 (3.6) | |
| State of disease, n (%) | n=58 | 0.06 | |||
| 1°RC | 21 (36) | 1 (12.5) | 10 (40) | 10 (40) | |
| 2°RC | 27 (46) | 7 (87.5) | 12 (48) | 8 (32) | |
| Active disease | 10 (17) | 0 | 3 (12) | 7 (28) | |
| Immunoglobulin g Cytomegalovirus R/D, n (%) | 0.07 | ||||
| R+/D− | 11 (15.7) | 4 (50) | 3 (8.82) | 4 (14.3) | |
| R+/D+ | 54 (77.2) | 4 (50) | 28 (82.4) | 22 (78.6) | |
| R−/D+ | 5 (7.1) | 0 | 3 (8.8) | 2 (7.1) | |
| Neutrophil engraftment time, days | 0 | ||||
| Median (Interquartile range) | 15.5 (13–2) | 20 (17–28) | 13.5 (12–16.3) | 17 (14.5–18.5) | |
| Platelet engraftment time, days | 0 | ||||
| Median (Interquartile range) | 16 (13–21) | 33 (20–59) | 14 (12.8–16.3) | 17 (15.5–23.5) | |
| Prevention strategy | 0.32 | ||||
| Preemptive therapy | 29 (41.4) | 2 (25) | 17 (50) | 10 (35.7) | |
| Prophylaxis | 41 (58.6) | 6 (75) | 17 (50) | 18 (64.3) | |
| Acute graft versus host disease | |||||
| Grade I–IV | 51 (72.8) | 6 (75) | 27 (79.4) | 18 (64.3) | 0.41 |
| Severe (III–IV) | 17 (24.2) | 3 (37.5) | 9 (26.5) | 5 (17.9) | 0.48 |
| EBMT score n=68 | 0.04 | ||||
| Zero-two | 25 (37) | 0 | 16 (48) | 9 (32) | |
| Three-seven | 43 (63) | 7 (100) | 17 (52) | 19 (68) | |
| Cytomegalovirus infection | 41 (58.6) | 7 (87.5) | 19 (55.9) | 15 (53.6) | 0.21 |
| Median (Interquartile range) transplant – viremia time-lapse, days | 51 (27–221)4–1331 | 27 (21–41)20–179 | 93 (32–278)4–1331 | 48 (28–253)10–1093 | 0.12 |
| Cytomegalovirus disease | 11 (15.7) | 4 (50) | 3 (8.8) | 4 (14.3) | 0.02 |
| Median (Interquartile range), monthsRange | 7 (3–19)0.3–44 | 3.8 (1.8–6.6)0.8–35 | 11.4 (4.9–18.2)1.2–44 | 5.6 (2.0–23.6)0.33–36 | 0.14 |
| Death | 43 (61.4) | 8 (100) | 18 (52.9) | 17 (60.7) | 0.05 |
| Cause of death | 0.89 | ||||
| Progression of the disease | 16 (37.2) | 3 (38) | 6 (33) | 7 (41) | |
| Transplant-related | 27 (62.8) | 5 (62) | 12 (67) | 10 (59) | |
| Cytomegalovirus infection-related | 7 (26) | 3 (60) | 1 (8.3) | 3 (30) | 0.08 |
The global pre-transplant risk was assessed according to the EBMT risk score; 57.1% of patients had a score between zero and four. Nevertheless, among the group of umbilical cord HSCT, 75% of patients had a score between five and seven.
In 77% of cases, the serology for CMV was a positive donor with a positive receptor. As a prevention strategy, 59% received prophylaxis with ganciclovir or valganciclovir and 41% of patients were monitored with the anticipated therapy strategy.
Analysis with the Mantel–Haenszel test showed that the risk of CMV disease varied when the preventive approach and the type of transplant were analyzed simultaneously, but this variation was not statistically significant (RR 1.2, 95% confidence interval (CI) 0.39–3.49, and adjusted RR 1.5, 95% CI 0.053–4.26).
When independently assessing the type of transplant, it was found that the risk of CMV disease appeared to be greater in patients with haploidentical transplant who had received preemptive therapy (RR 5.4, 95% CI 0.64–45.03). Nevertheless, the opposite was found for identical transplant, with preemptive therapy being a protective factor in this case (RR 0.5, 95% CI 0.049–5.00).
The risk of CMV disease, according to the EBMT score and preventive approach, showed that the risk of disease with an EBMT score >3 (RR 1.57, 95% CI 1.117–2.1) was not affected by the preventive strategy used (adjusted RR 1.57, 95% CI 1.18–2.09). Overall, there were no differences in the risk among patients who received preemptive therapy and those who received prophylaxis (RR 1.1, 95% CI 0.54–2.3).
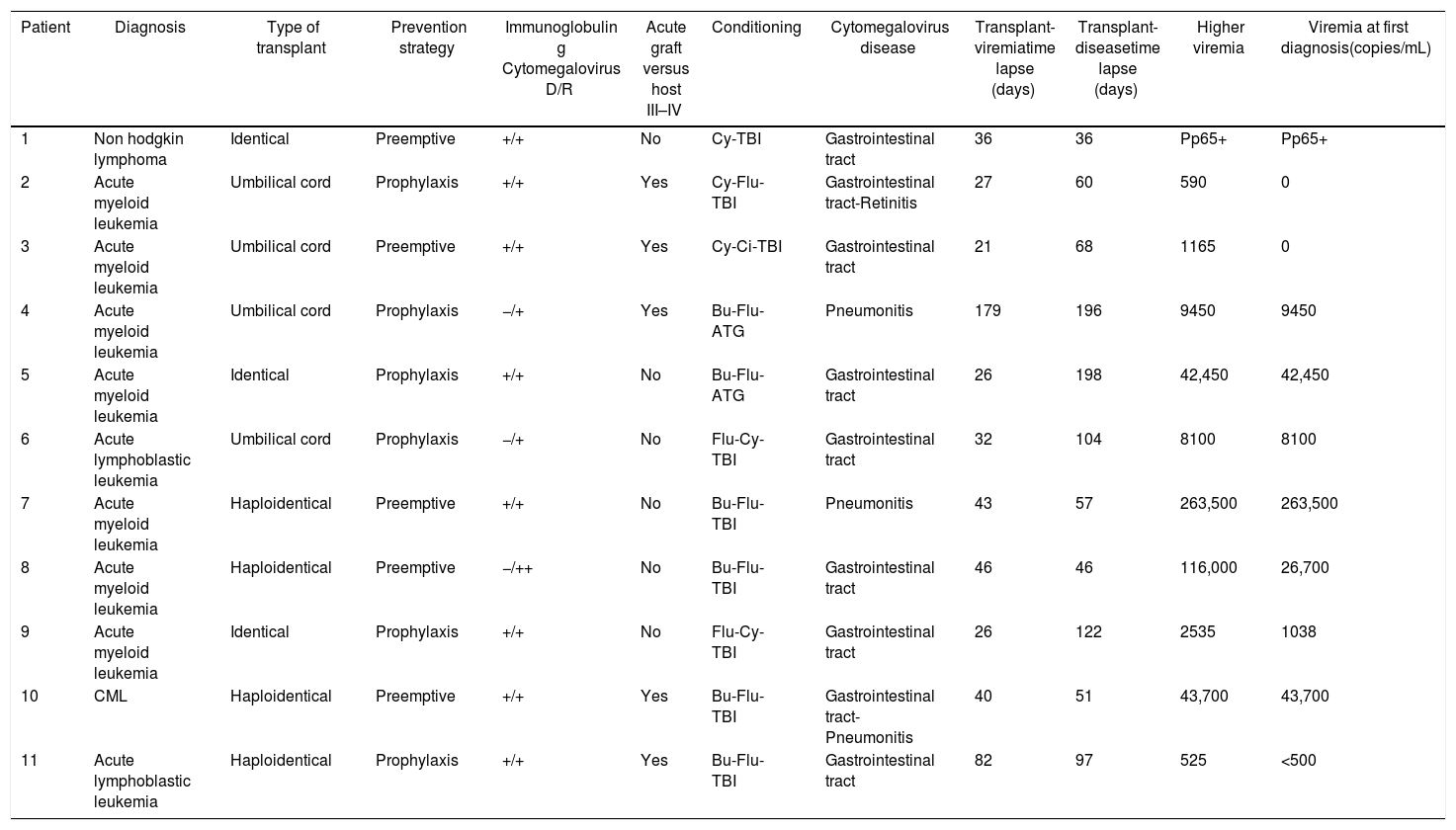
A total of 59% of the patients presented infection, with a median of 51 days. CMV disease was diagnosed in 11 patients (16%), and the clinical characteristics of these patients are presented in Table 2.
Clinical characterization of patients who developed CMV disease.
| Patient | Diagnosis | Type of transplant | Prevention strategy | Immunoglobulin g Cytomegalovirus D/R | Acute graft versus host III–IV | Conditioning | Cytomegalovirus disease | Transplant-viremiatime lapse (days) | Transplant-diseasetime lapse (days) | Higher viremia | Viremia at first diagnosis(copies/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Non hodgkin lymphoma | Identical | Preemptive | +/+ | No | Cy-TBI | Gastrointestinal tract | 36 | 36 | Pp65+ | Pp65+ |
| 2 | Acute myeloid leukemia | Umbilical cord | Prophylaxis | +/+ | Yes | Cy-Flu-TBI | Gastrointestinal tract-Retinitis | 27 | 60 | 590 | 0 |
| 3 | Acute myeloid leukemia | Umbilical cord | Preemptive | +/+ | Yes | Cy-Ci-TBI | Gastrointestinal tract | 21 | 68 | 1165 | 0 |
| 4 | Acute myeloid leukemia | Umbilical cord | Prophylaxis | −/+ | Yes | Bu-Flu-ATG | Pneumonitis | 179 | 196 | 9450 | 9450 |
| 5 | Acute myeloid leukemia | Identical | Prophylaxis | +/+ | No | Bu-Flu-ATG | Gastrointestinal tract | 26 | 198 | 42,450 | 42,450 |
| 6 | Acute lymphoblastic leukemia | Umbilical cord | Prophylaxis | −/+ | No | Flu-Cy-TBI | Gastrointestinal tract | 32 | 104 | 8100 | 8100 |
| 7 | Acute myeloid leukemia | Haploidentical | Preemptive | +/+ | No | Bu-Flu-TBI | Pneumonitis | 43 | 57 | 263,500 | 263,500 |
| 8 | Acute myeloid leukemia | Haploidentical | Preemptive | −/++ | No | Bu-Flu-TBI | Gastrointestinal tract | 46 | 46 | 116,000 | 26,700 |
| 9 | Acute myeloid leukemia | Identical | Prophylaxis | +/+ | No | Flu-Cy-TBI | Gastrointestinal tract | 26 | 122 | 2535 | 1038 |
| 10 | CML | Haploidentical | Preemptive | +/+ | Yes | Bu-Flu-TBI | Gastrointestinal tract-Pneumonitis | 40 | 51 | 43,700 | 43,700 |
| 11 | Acute lymphoblastic leukemia | Haploidentical | Prophylaxis | +/+ | Yes | Bu-Flu-TBI | Gastrointestinal tract | 82 | 97 | 525 | <500 |
Acute GVHD was seen in 51 patients (73%), however, only 24% presented grade III-IV GVHD. In 5/17 (29%) the diagnosis of GVHD was prior to the diagnosis of CMV disease, which represents 45% of all patients who had CMV disease.
The median follow-up was 7 months (interquartile range (IQR) 3–19). A total of 43 patients of the cohort (61%) died, 37% of deaths were related to progression of the disease, and 63% were transplant-related. The mortality among patients who developed CMV disease was 91%, mostly due to the CMV infection (70%), followed by GVHD, with or without associated bacterial infection (30%).
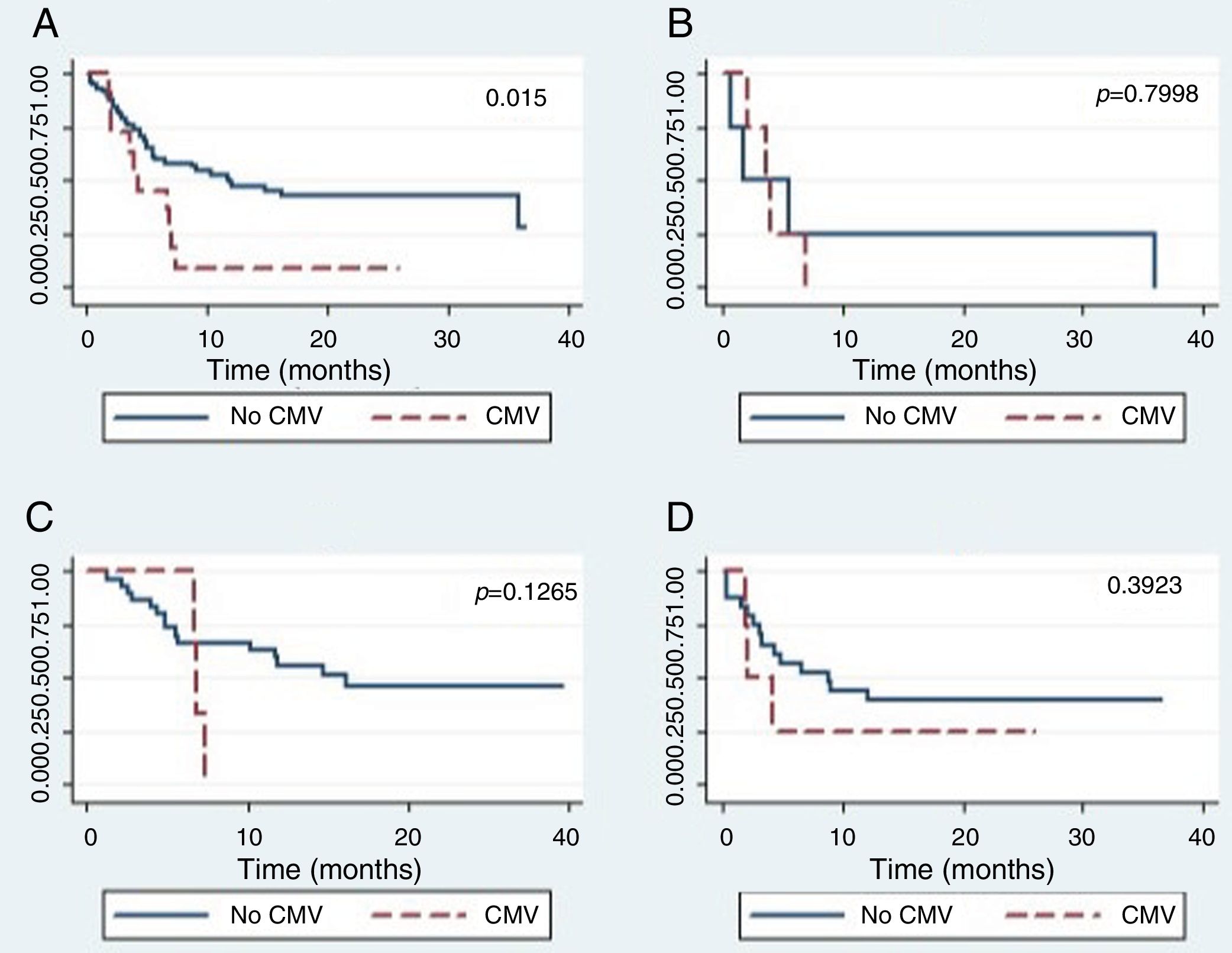
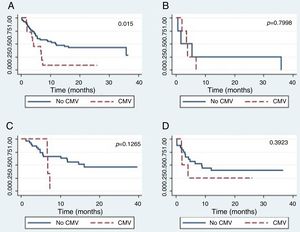
The global survival after one-year post-HSCT in patients who had not developed CMV disease was 44% (CI 24–62), while for patients who developed CMV disease, it was 25% (CI 0–66), which was a statistically significant difference (p=0.015) (Figure 2.) The global survival after one-year post-HSCT in patients who had not developed CMV infection was 48%, the IC 28–65%, versus 39% with an IC 24–53% in patients who developed CMV infection (p=0.5067).
There were no differences in the global survival rate related to CMV disease among the different types of transplants.
The 100-day mortality was 25%, and transplant-related mortality was 19.5%.
DiscussionIn this study, an assessment of CMV infection among HSCT patients over a period of 8 years was performed, comparing the population in terms of the type of transplant: haploidentical, identical, or umbilical cord. There were no differences among the groups in terms of the conditioning regimen and immunosuppression, making them comparable.
The incidence of CMV infection was 59%, which is consistent with previous literature reports on incidence, that oscillate between 34% and 90%. This wide range is attributable to populational differences in terms of CMV serostatus prior to transplantation, conditioning protocols, and laboratory techniques employed in the diagnosis.6 The only known report for Latin America in which patients with HSCT were included was made by Bonon and collaborators and the results showed an incidence of infection of 69% and an incidence of disease of 11%. The cohort included had serological characteristics similar to ours.7
A total of 16% of our patients developed CMV disease, being the gastrointestinal tract the end-organ most frequently affected, followed by pneumonitis and a case of retinitis. The median time of onset of the disease was 94 days post-HSCT, which matches previous reports that describe the entity being more frequent in the first 3 months post-HSCT, being associated with the immunosuppression status.8
The incidence of disease in our group was significantly high compared to that published by other authors. In the cohort evaluated by Peres et al., only 6.7% developed the disease, and in the study performed by Bonon, this event was present in 8.7%. However, in these analyses only identical donors were included, as well as a low incidence of GVHD (40%).7,9
Multiple studies have found that the risk of developing CMV disease, is directly proportional to the level of the viral load.10,11 Nevertheless, in this study CMV disease developed even in patients with small viral loads, demonstrating the importance of initiating antiviral therapy early on.
The CMV infection and disease were, in proportion, more frequent among the cord transplant group. Albano et al. reported an overall CMV infection incidence of 23%12 to the American registry of cord transplant. In contrast, in the Chinese registry, there was an incidence of CMV infection of 71%.13
During four years, at Fundacion Valle del Lili, umbilical cord stem cells were used as an alternative for those patients without a related identical donor. The presence of multiple complications was evident in this group, including CMV infection, related to the delayed immune reconstitution seen in this type of transplant.
The risk analysis failed to statistically demonstrate that the type of transplant has an effect modification on the risk for CMV disease among the different prevention strategies; the latter was evident by the inconclusive confidence intervals, and it is possibly attributable to the small number of events.
The risk for CMV disease was not affected by the adopted prevention regimen. The clinical trial conducted by Boeckh et al. concluded that prophylaxis did not improve CMV-free and invasive infection-free survival, when compared to preemptive therapy and no statistically significant differences were observed in secondary descents.14 This finding is important because the long-term CMV prophylaxis is poorly tolerated and may be related to myelotoxicity. We believe that a study should be conducted with more patients to confirm that there are no differences between the prophylaxis strategies, since this could impact the costs of the transplant.
The CMV has a great impact on the survival of transplant patients. The overall survival was lower for the group that developed CMV disease, with a statistically significant difference. These data are comparable to previous studies in which this condition considerably affects survival.11,15 Mortality in these patients is associated not only with infection by the virus, but we also found that these patients had acute grade III–IV GVHD, associated with immunosuppression, which in many cases conditioned the development of severe bacterial infections.
The limitations of this study include the retrospective design, which made it difficult to access the information collected in the medical records. We consider that there are unmeasured variables, such as adherence to antiviral treatment, that may influence the presentation of outcomes. In addition, until 2011 the infection of CMV was measured with the pp65 antigen and this test is less sensitive than the PCR; it has been found that antigenemia can miss 35% of CMV infections tested by PCR.16
This study confirms that, despite new advances in the transplantation of hematopoietic stem cells, infection is frequent within the first 100 days, and the development of CMV disease occurs at any level of viral load.
Conflicts of interestThe authors declare no conflicts of interest.