Hairy Cell Leukemia (HCL) is a rare, indolent, B-lymphoproliferative disorder, which represents approximately 2–4% of lymphoid leukemias. Patients are diagnosed at a mean age of 55–60 years and there is a clear male predilection, with a ratio of 5:1.1,2
HCL was first described in 1958 and, at that time, patients had a poor prognosis, with splenectomy being the standard of care, even though it only provided short periods of remission.1 In 1984, interferon-alpha (IFN-α) emerged as a promising treatment, with complete remission being described in up to 40% of cases and some type of response seen in up to 90% of cases.3 Due to its side effects, inconvenient route of administration and in an attempt to improve responses for a larger number of patients, other therapies emerged as substitutes for IFN-α, including cladribine and, currently the BRAF inhibitor vemurafenib.1
Based on data from the literature and personal unsuccessful previous experiences, cladribine should not be used to treat HCL patients presenting with active infection.4 Nevertheless, a striking point is the improvement of blood cell counts in critically ill patients. It seems fundamental to make clinicians and hematologists aware of the hazard of intensive chemotherapy in this group of patients and the role of IFN-α, a commonly forgotten therapy albeit widely available. IFN-α, with its low toxicity profile, can be used in cases to increase blood cell counts.
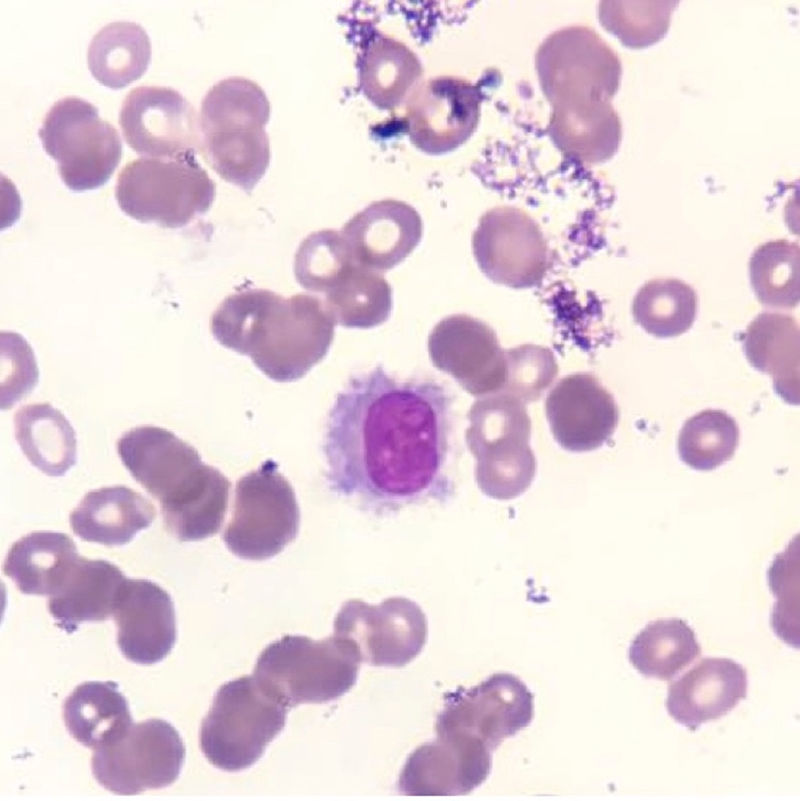
Case reportAn informed consent form was signed by the patient. A 48-year-old previously healthy man complained of asthenia, daily fever, night sweats and a weight loss of 6kg over three months. He was admitted to the emergency room complaining of cough and dyspnea on exertion in the previous five days. A blood cell count had been performed in another service one week earlier, which showed pancytopenia including severe neutropenia (hemoglobin: 6.0g/dL; leukocyte count: 0.8×109/L, neutrophil count: 0.14×109/L, lymphocyte count: 0.48×109/L, monocyte count: 0.17×109/L and platelet count: 70.0×109/L). Physical examination did not demonstrate any adenopathy or hepatosplenomegaly. His family history was negative for hematological disorders or any other neoplasm. A presumptive diagnosis of bacterial pneumonia was made and the patient was hospitalized in a semi-intensive care unit. All blood cultures were negative as was a serum galactomannan test. A bone marrow aspirate was also performed, which demonstrated 36% of lymphoid cells with morphology resembling hairy cells (Figure 1). Immunophenotyping by flow cytometry revealed expression of CD19, CD20bright, CD11c, CD25, CD103, CD123 and monoclonality demonstrated by light chain restriction (lambda), confirming a classic HCL. Morphological aspects and CD25/CD123 expression helped to exclude HCL variants and splenic lymphoma. A bone marrow biopsy could not be performed due to restricted movement because of the diagnosis of acute respiratory distress syndrome (ARDS).
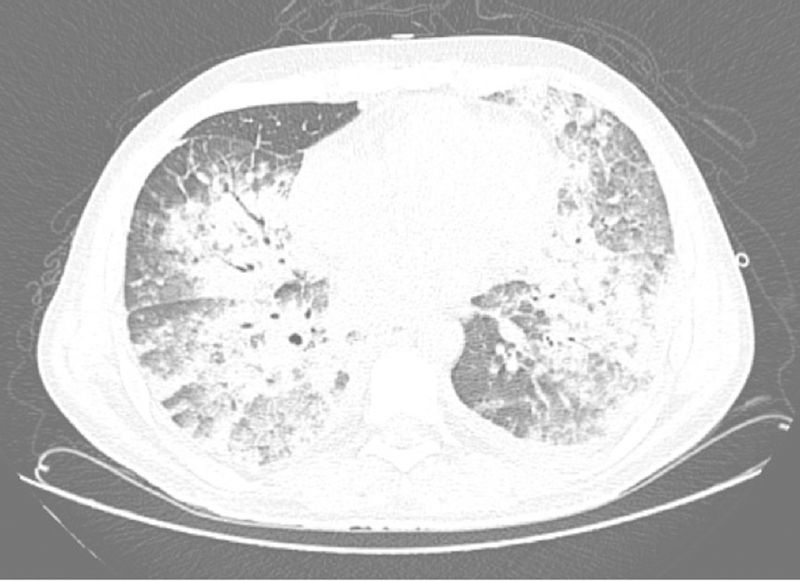
A week after the diagnosis, despite the use of broad-spectrum antibiotics (piperacillin/tazobactam and vancomycin), his general condition deteriorated, with significant worsening of the dyspnea, persistent fever, and then the patient progressed to septic shock of pulmonary origin associated with ARDS and acute renal injury requiring hemodialysis (Figure 2). Orotracheal intubation was indicated at this moment. Bacterial and fungal cultures of a tracheal aspirate were performed but were also negative. Since an infectious etiology was not recognized, his antibiotic regimen was broadened to meropenem and liposomal amphotericin B, maintaining vancomycin for gram-positive coverage. Subcutaneous Filgrastim was also given as an attempt to raise the neutrophil count.
Concurrently, due to this important clinical deterioration, severe infectious complication and deep neutropenia, it was decided to start IFN-α at 3,000,000 IU three times a week, based on a formal contra-indication to intensive chemotherapy (cladribine) and the unavailability of vemurafenib in our center. The BRAF V600E mutation, a genetic marker for classical HCL, was investigated by pyrosequencing in peripheral blood, but the result was negative. It is worth remembering that at this moment the patient had a low lymphocyte count and was already receiving IFN-α.
After five days of medication and intensive care, the patient started progressive weaning of vasoactive drugs and was extubated, while his neutrophil count was increasing. Filgrastim was continuously administered for seven days up to this moment and two packed red blood cell units had been provided since hospital admission. Blood counts after six days of medication had evolved (hemoglobin: 8.2g/dL; leukocyte count: 2.68×109/L; neutrophil count: 1.1×109/L; lymphocyte count: 1.4×109/L; monocyte count: 0.1×109/L; platelet count: 0.63×109/L). He progressively recovered his status performance and renal function and was later able to receive a more intensive regimen to treat his disease.
After a complete recovery of clinical status and resolution of acute renal failure, cladribine (0.09mg/kg/day) was prescribed as a continuous intravenous infusion for seven days without significant toxicity and the patient was discharged to outpatient follow-up. To date, he presents complete recovery of his blood counts and is in a satisfactory general condition, with a good quality of life. A bone marrow biopsy was performed six months after the cladribine regimen confirming a complete response.
DiscussionHCL is a rare disease which usually presents as asymptomatic pancytopenia or with B symptoms arising from cellular proliferation and hypercytokinemia.1 Early clinical studies demonstrated a significant increase in the risk of infections in these patients due to the serious defect of the monocyte-macrophage lineage and a decrease in both myeloid and lymphoid dendritic cell counts.5,6
Diagnosis of HCL is made by a combination of clinical, morphological, histopathological and immunophenotypic aspects. Recently, the BRAF V600E mutation was repeatedly identified in patients with HCL and, besides supporting the diagnosis, contributes to the elucidation of pathogenic mechanisms and may have a prognostic role in a subset of patients.1 In this case, the BRAF mutation, was probably negative by pyrosequencing due to the low number of circulating malignant cells; allele-specific polymerase chain reaction or next-generation sequencing techniques are more sensitive for this type of sample.1
Prior to the introduction of IFN-α and purine nucleosides, infectious complications were a common part of the natural history of HCL. These infections often involved both gram-positive and gram-negative organisms as well as Aspergillus and other fungi. Golomb & Hadad studied 127 patients and found 47 (37%) with documented infections and an additional 40 (31%) with presumed infections.6 Twenty-nine of the 47 patients died because of these infections. Another series of 22 patients identified 18 life-threatening infections.7 Mycobacterial infections are another group of infections that must be considered in respect to these patients.8 As demonstrated by Damaj et al., lymphopenia at diagnosis may be a risk factor for infections, even late in the course of disease.5 Our patient presented with a noticeable lymphopenia (0.48×109cells/L).
Except for the French recommendation to manage HCL, other recent guidelines have not made it clear what the ideal approach to patients diagnosed with HCL and active infection are.1,4,9 All of them clearly state the formal contra-indication of purine analogs in these patients, especially among those who develop other complications such as renal failure or ARDS, as seen in the present case. The role of hematopoietic growth factors such as filgrastim has not been established, since the result might be rather variable.
Although in this case we cannot firmly state that IFN-α was the only reason for neutrophil recovery, according to the available literature and our previous experience, we really believe that IFN-α had a pivotal role.
Despite the unavailability of the BRAF inhibitor vemurafenib in the public sector in Brazil, this drug is another recently recognized option for HCL patients who present with active infections, since it is not very myelosuppressive and presents a manageable profile of side effects.9 Despite limited evidence, pentostatin, another locally unavailable purine analog with low myelosuppression, can be administered at lower doses in patients with active infections or bleeding, thus reducing treatment-related morbidity and mortality.10
Reports of long-term results of IFN-α are widely available in the literature, but it seems important to mention its role in the early treatment of HCL especially in patients presenting very severe neutropenia (<0.2×109cells/L) and/or active infection, which may be difficult to control due to profound cellular immunosuppression. IFN-α functions as a cytostatic agent in HCL and is usually well tolerated at the doses and schedule prescribed in patients with HCL, besides being a less expensive option for these patients compared to vemurafenib or pentostatin.11
Conflicts of interestThe authors declare no conflicts of interest.