This is a quantitative transversal study that aimed to analyze the sociodemographic and clinical characteristics of thalassemia major patients with and without diabetes mellitus.
MethodThe cohort consisted of 31 thalassemia major patients from a reference center of treatment in Brazil in 2016. The data were obtained from an interview using a questionnaire containing demographic and clinical variables. The results show that 16.1% of the participants with thalassemia major had diabetes mellitus. The participants’ ages ranged from 20 to 48 years, with an average of 35 years, mostly students and starting in the formal job market. The most commonly used treatment was the oral desferasirox and the transfusion treatment interval was 15–22 days.
ResultsPatients with thalassemia major and diabetes mellitus presented altered values of fasting glycemia, serum alanine transaminase, magnetic resonance imaging and bone densitometry.
ConclusionIt was concluded that knowledge of the characteristics of this population contributes in the proposal of effective educational strategies in light of the complexity of care and the progression of the disease.
Hemoglobinopathies are genetically determined inherited disorders of human hemoglobin, with significant morbidity worldwide.1 Among the hemoglobinopathies there are the alpha and beta thalassemia – major, intermediate and minor – and sickle cell diseases. These types of hemoglobinopathies have anemia and a broad spectrum of clinical severity in common. The severity of anemia and its clinical consequences depend on the molecular defects of each individual.2
Among those with greater severity, the patients depend on regular blood transfusions for their survival, as in beta thalassemia major.2,3 Patients with beta thalassemia major (TM) without appropriate treatment will develop severe anemia that can lead to organic and toxic modifications with serious implications for health and even death.4
Still, TM patient records are incomplete. In 2013, the Thalassemia International Federation (TIF), showed that the prevalence of major and intermediate thalassemia in seven European countries was high, the total number being over 17,000 cases. Similar sources without controlled records have shown that in the Eastern Mediterranean Region it has been estimated there are 108,000 patients with thalassemia.2
In Brazil in 2015, the General Coordination of Blood and Hemoderivatives of the Department of Specialized and Thematic Care of the Healthcare Office and the Health Ministry, in partnership with the members of the Technical Advisory Committee, performed a study to identify patients with thalassemia major in hematology and hemotherapy services. This study showed that 305 patients had thalassemia major, with the majority concentrated in the Southeast region (66.56%), followed by the South (12.91%), Northeast (11.59%), Midwest, (28%) and North (1.66%).4
The treatment consisting in red blood cell transfusion is performed repeatedly at appropriate intervals, causing inhibition of inefficient erythropoiesis, allowing the patient to function properly, with an effective quality of life. An iron chelator is also used, administered parenterally or orally, which leads to changes in the patient's lifestyle and difficulties in adhering to the treatment, and this leads to the accumulation of iron in different organs in the organism, such as the liver, heart, pancreas, endocrine glands and bone marrow, possibly contributing to the onset of other diseases, such as diabetes mellitus (DM).3,5
Diabetes mellitus in TM patients is complex and multifactorial. The initial factor can affect insulin resistance mediated by iron, instead of the defective production of insulin, which consequently damages the pancreatic beta cells. It has also been shown that an insulin secretion defect of β cells can be present early, before the development of glucose intolerance, resulting from toxic effects of iron deposition in the pancreas.6–9
In addition, insulin deficiency, chronic liver disease, viral infection and/or genetic factors may also be contributory factors in the development of DM in patients with thalassemia major.3 The prevalence of DM in TM patients ranges from 9.7% to 29%.6–9
In Brazil there is still a shortage of studies regarding sociodemographic and clinical characteristics of TM and DM patients. In view of the above, this study aims to analyze the sociodemographic and clinical characteristics of patients with thalassemia major with and without DM.
MethodologyThis was a quantitative cross-sectional study performed at a referral center in the treatment of transplanted, hemophiliac and hereditary anemias patients in the State of São Paulo, Brazil, in 2016. The study population consisted of 33 patients with thalassemia major at the Center. All of them were invited to participate in the study, but two patients were refused. Thus, the study cohort consisted of 31 patients with thalassemia major. To collect data, a survey containing demographic variables (age, sex, family income and occupation) and clinical variables (time of diagnosis, other diseases, treatment and values of fasting glucose, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum ferritin, bone densitometry, abdominal ultrasound and liver and heart resonance imaging). The data were collected through directed interviews and consultation of health records, from June to August 2015. For the analysis, the data were double-typed in the database in the Microsoft Excel program and validated. They were then imported into the Statistical Package for the Social Sciences (SPSS) for Windows, version 17.0. Descriptive statistical analysis was performed using frequency and percentage tables. To better visualize the results, we present the data in two patient groups, with thalassemia major without DM (Thal-no DM) and with DM (Thal-DM). The project was approved by the Research Ethics Committee (CAAE) under number 41912415.3.0000.5393.
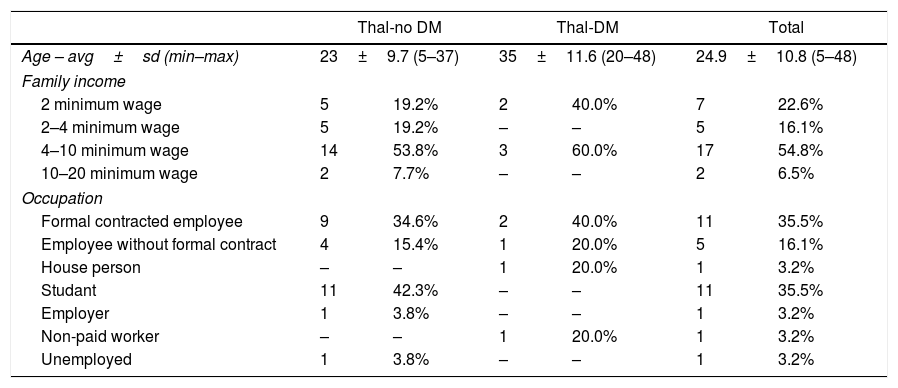
ResultsOf the 31 TM patients, 15 were female and 16 were male, 30 were single and 10 had completed high school. The age ranged from 5 to 48 years, average of 24.9 years. Of the 31 patients investigated, 5 had DM. Of these, the age ranged from 20 to 48 years, average of 35 years.
Table 1 shows the numerical and percentage distribution of patients in the groups Thal-no DM and Thal-DM, according to sociodemographic variables.
Numerical distribution and percentage of thalassemia major patients with and without diabetes mellitus, according to age, family income and occupation. Ribeirão Preto, 2015.
| Thal-no DM | Thal-DM | Total | ||||
|---|---|---|---|---|---|---|
| Age – avg±sd (min–max) | 23±9.7 (5–37) | 35±11.6 (20–48) | 24.9±10.8 (5–48) | |||
| Family income | ||||||
| 2 minimum wage | 5 | 19.2% | 2 | 40.0% | 7 | 22.6% |
| 2–4 minimum wage | 5 | 19.2% | – | – | 5 | 16.1% |
| 4–10 minimum wage | 14 | 53.8% | 3 | 60.0% | 17 | 54.8% |
| 10–20 minimum wage | 2 | 7.7% | – | – | 2 | 6.5% |
| Occupation | ||||||
| Formal contracted employee | 9 | 34.6% | 2 | 40.0% | 11 | 35.5% |
| Employee without formal contract | 4 | 15.4% | 1 | 20.0% | 5 | 16.1% |
| House person | – | – | 1 | 20.0% | 1 | 3.2% |
| Studant | 11 | 42.3% | – | – | 11 | 35.5% |
| Employer | 1 | 3.8% | – | – | 1 | 3.2% |
| Non-paid worker | – | – | 1 | 20.0% | 1 | 3.2% |
| Unemployed | 1 | 3.8% | – | – | 1 | 3.2% |
Regarding the clinical variables, the predominant disease time was 21–30 years in 16 patients. For most, the diagnosis was made in the first months of life up to 6 years of age; for 11 patients, thalassemia was diagnosed at 12 months, and; at 18, up to 3 years. Of the 5 Thal-DM patients, 3 had received the diagnosis of DM five years ago and only 1 had had the diagnosis made more than 30 years ago.
Regarding the treatment of Thal-no DM patients, 19 used oral deferasirox. Of the Thal-DM patients, 2 used a combined therapy of deferoxamine and deferiprone. Insulin was used by 4 patients. The time interval for transfusion treatment in the two groups ranged from 15 to 22 days.
Regarding the other above-mentioned diseases, of the 31 Thal-no DM patients, 9 reported more than one comorbidity, 1 with arterial hypertension, 7 with hepatosplenomegaly, and 9 with splenomegaly. Of the 5 Thal-DM patients, 2 reported osteoporosis.
In relation to the laboratory test results, 2 Thal-no DM and 2 Thal-DM patients presented altered fasting glycemia. Regarding serum ferritin, only 5 of the Thal-no DM patients presented results within normal values, as did 3 Thal-DM patients. Regarding creatinine, 15 Thal-no DM patients presented adequate control and 4 Thal-DM patients presented values below normal. Regarding the AST and ALT values, 14 Thal-no DM patients presented adequate control. Four Thal-DM patients had adequate control for AST, and 3 had inadequate ALT (Table 2).
Numerical distribution and percentage of patients with thalassemia major, with and without diabetes mellitus, according to fasting glycemia, serum ferritin, serum creatinine, aspartate aminotransferase and alanine aminotransferase. Ribeirão Preto, 2015.
| Variable | Thal-no DM | Thal-DM | Total | |||
|---|---|---|---|---|---|---|
| Exams | n | % | n | % | n | % |
| Fasting glycemia with DM | – | – | 5 | 100 | 5 | 100 |
| <70mg/dL hypoglycemia | – | – | 1 | 20.0 | 1 | 20.0 |
| <110mg/dL adequate control | – | – | 1 | 20.0 | 1 | 20.0 |
| ≥110 and ≤130mg/dL tolerable metabolic control | – | – | 1 | 20.0 | 1 | 20.0 |
| >130mg/dL inadequate control | – | – | 2 | 40.0 | 2 | 40.0 |
| Fasting glycemia without DM | 26 | 100 | – | – | 26 | 100 |
| >70 and <100mg/dL adequate control | 24 | 92.3 | – | – | 24 | 92.3 |
| ≥100 and <126mg/dL altered fasting glycemia | 2 | 7.7 | – | – | 2 | 7.7 |
| ≥126mg/dL diabetes mellitus | – | – | – | – | – | – |
| Ferritin | 26 | 100 | 5 | 100 | 31 | 100 |
| <1000μg/L recommended | 5 | 19.2 | 3 | 60.0 | 8 | 25.8 |
| 1000–2500μg/L light overload | 12 | 46.2 | 2 | 40.0 | 14 | 45.2 |
| >2500μg/L serious overload | 7 | 26.9 | – | – | 7 | 22.6 |
| ≥7500μg/L non-quantified result | 2 | 7.7 | – | – | 2 | 6.5 |
| Creatinin | 26 | 100 | 5 | 100 | 31 | 100 |
| <0.6mg/dL below normality | 10 | 57.7 | 4 | 80.0 | 14 | 45.2 |
| 0.6 and 1.3mg/dL adequate control | 15 | 62.5 | 1 | 20.0 | 16 | 51.6 |
| >1.4mg/dL inadequate control | 1 | 3.8 | – | – | 1 | 3.2 |
| AST | 26 | 100 | 5 | 100 | 31 | 100 |
| <32u/L adequate control | 14 | 53.8 | 4 | 80.0 | 18 | 58.1 |
| >33u/L inadequate control | 12 | 46.2 | 1 | 20.0 | 13 | 41.9 |
| ALT | 26 | 100 | 5 | 100 | 31 | 100 |
| <31u/L adequate control | 14 | 53.8 | 2 | 40.0 | 16 | 51.6 |
| >32u/L inadequate control | 12 | 46.2 | 3 | 60.0 | 15 | 48.4 |
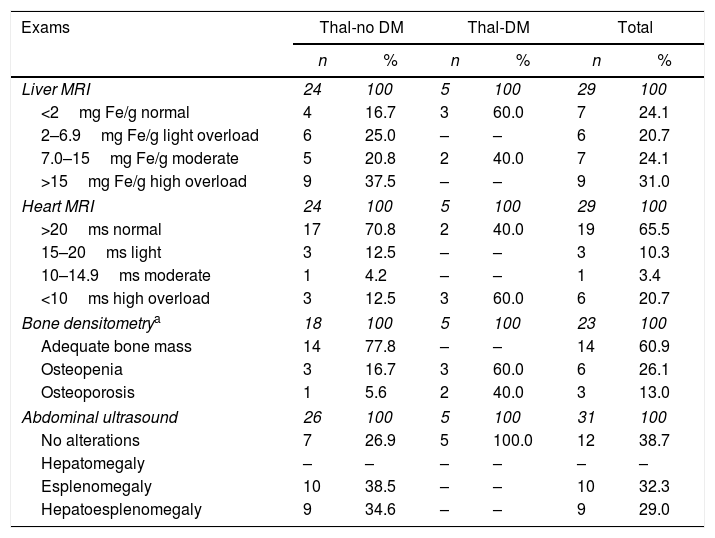
Regarding the liver resonance exams, 4 Thal-no DM patients presented normal levels. In the Thal-DM group 3 presented normal results. Regarding the MRI of the heart, 17 Thal-no DM patients presented values within the parameters of normality. Of the Thal-DM group, 3 presented cardiac iron accumulation values considered serious (Table 3).
Numerical distribution and percentage of patients with thalassemia major, with and without diabetes mellitus, according to magnetic resonance imaging of the liver and heart, bone densitometry and abdominal ultrasound. Ribeirão Preto, 2015.
| Exams | Thal-no DM | Thal-DM | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Liver MRI | 24 | 100 | 5 | 100 | 29 | 100 |
| <2mg Fe/g normal | 4 | 16.7 | 3 | 60.0 | 7 | 24.1 |
| 2–6.9mg Fe/g light overload | 6 | 25.0 | – | – | 6 | 20.7 |
| 7.0–15mg Fe/g moderate | 5 | 20.8 | 2 | 40.0 | 7 | 24.1 |
| >15mg Fe/g high overload | 9 | 37.5 | – | – | 9 | 31.0 |
| Heart MRI | 24 | 100 | 5 | 100 | 29 | 100 |
| >20ms normal | 17 | 70.8 | 2 | 40.0 | 19 | 65.5 |
| 15–20ms light | 3 | 12.5 | – | – | 3 | 10.3 |
| 10–14.9ms moderate | 1 | 4.2 | – | – | 1 | 3.4 |
| <10ms high overload | 3 | 12.5 | 3 | 60.0 | 6 | 20.7 |
| Bone densitometrya | 18 | 100 | 5 | 100 | 23 | 100 |
| Adequate bone mass | 14 | 77.8 | – | – | 14 | 60.9 |
| Osteopenia | 3 | 16.7 | 3 | 60.0 | 6 | 26.1 |
| Osteoporosis | 1 | 5.6 | 2 | 40.0 | 3 | 13.0 |
| Abdominal ultrasound | 26 | 100 | 5 | 100 | 31 | 100 |
| No alterations | 7 | 26.9 | 5 | 100.0 | 12 | 38.7 |
| Hepatomegaly | – | – | – | – | – | – |
| Esplenomegaly | 10 | 38.5 | – | – | 10 | 32.3 |
| Hepatoesplenomegaly | 9 | 34.6 | – | – | 9 | 29.0 |
Regarding the bone densitometry exams, 14 Thal-no DM patients had adequate bone mass and 8 had no record of this examination in their health records. Of the Thal-DM patients, 3 had osteopenia. Regarding abdominal ultrasound, 10 and 9 patients presented with splenomegaly and hepatosplenomegaly, respectively. Five Thal-DM patients presented abdominal ultrasound examination results within the parameters of normality (Table 3).
DiscussionThe prevalence of TM patients with DM was 16.1%. The prevalence rate of DM in TM patients in the literature varies from 9.7% to 29%.6–9 In a study conducted in China in 2008, 72 patients with TM showed a higher rate (29.0%).8 Another study conducted in Egypt in 2010 with 40 TM patients also showed a high rate (25.0%).9 However, these results are not comparable, as the studies in China and Egypt included patients aged over 12 years and under 18 years, respectively. The prevalence of DM patients in the TM population in different regions of the world has been increasing. Thus, the monitoring of the TM patient from the earliest years of life, by a specialist in the field of endocrinology, may prevent or delay the early onset of the disease.
As for the sex, the results obtained are similar to the distribution reported in the literature,9–11 that is, the prevalence is similar in men and women, since the genetic defect comes from both the mother and the father. On the other hand, there are studies that show that TM is more prevalent in women.8,12,13
The average age of the participants was 24.9 years. Currently there is an increase in the life expectancy of the TM population due to the appearance of iron chelators in the treatment.4 It should be noted that patients before the emergence of new treatments lived until the second decade of life. The importance of tracing a parallel age of TM patients over the years favors the monitoring of each stage of the patient's development effectively, as well as further studies for this population.
When analyzing the results obtained related to occupation, it was discovered that 29 (93.5%) patients were inserted in the work market or they were students, according to a study performed in Greece showing that the majority of the patients had some occupation related to study or work.13 Participation in the job market, performance in paid activities, increased level of schooling and the desire to set up a family are expectations of TM patients. They are particularly susceptible in late adolescence and early adulthood to these aspirations, as well as questions about sexual intercourse, career development, and financial dependence.14 Our results show that patients with TM are inserted in the labor market and in professional training institutions and are seeking to develop their activities in the daily life, despite the presence of other diseases and obstacles in the compliance with the therapeutic plan.
Concerning the treatment of TM, the type of chelator most used was oral deferasirox for 20 (64.5%) patients and the use of combined therapy for 4 (12.9%). The substitution of the administration route of the chelator from subcutaneous to oral has been increasing over the last years, according to a study done in France, where the oral chelator administration rate is 75.5%, and the use of combination therapy, 4%.15
As for the presence of other diseases reported by TM patients, similar results were obtained with respect to hepatosplenomegaly. However, other studies also highlight heart problems and hypothyroidism.8,13–15 In this study, there was a case of cardiopathy and hypothyroidism declared by the patient. A study conducted in China in 2011 with 363 TM patients found a high prevalence of endocrine failure, with DM occurring in 25% of TM adults. This data shows that DM emerges as cause of morbidity in patients with TM.7
When analyzing the values of laboratory tests, Thal-DM patients presented serum ferritin values at more adequate levels than Thal-no DM patients. Furthermore, a study developed in Egypt with thalassemic patients with and without DM showed serum ferritin results higher in patients with DM.9 The average serum ferritin found in the literature for thalassemic patients without DM was higher than advisable, being in some cases considered as a serious iron overload.9,12,15
The divergences of data obtained from the literature can be explained by considering that iron overload can cause complications in the body. In this manner, the patient's adherence to the drug treatment, transfusion therapy and systematic monitoring of the evolution of the disease through clinical, laboratory and complementary exams is necessary.
Regarding the magnetic resonance imaging of the liver, no Thal-DM patients presented severe hepatic iron overload, however in the Thal-no DM group, 20 (83%) presented some overload, be it light or severe. A study conducted in Egypt with 40 patients showed elevated levels of hepatic and pancreatic iron.9 Another study conducted in London with 22 TM and DM patients showed that almost half had a severe iron overload.12 Regarding the heart, 17 (70.8%) Thal-no DM patients presented results within the normal parameters and 3 (60.0%) Thal-DM patients presented a serious cardiac overload. These results reinforce the importance of continuous monitoring of TM and DM patients throughout the treatment.
It is recognized that the advancement of methods and techniques of iron and chelation evaluation may contribute to a reduction in cardiac morbidity, having a future impact on the health of thalassemia major patients. One study showed that the iron overload in the pancreas cannot be assessed solely by the patient's age and by serum ferritin values. This study stressed that the accumulation of pancreatic iron is not correlated with the accumulation of liver iron, but that there is a strong correlation between the accumulation of iron in the pancreas and the heart.8 For this purpose, pancreatic iron overload can be evaluated by magnetic resonance imaging, but iron accumulation in other organs did not correlate significantly with pancreatic hemosiderosis.16 At the center of our study, only liver and heart resonance is performed.
This advance allows early detection of iron overload in the pancreas, with a view toward reorienting patient care and proposing effective preventive actions to delay the onset of DM. For Thal-DM patients, magnetic resonance imaging of the pancreas and the systematic evaluation of fasting glycemia may contribute to good disease control, prevention of complications and consequently, improvement in quality of life.
Regarding bone densitometry, the majority of Thal-no DM patients had adequate bone mass. In Thal-DM patients, there was an increase in osteopenia and osteoporosis, which was in agreement with other studies.7,12 The monitoring of thalassemic patients favors early detection of osteopenia and osteoporosis, some of the most common bone complications of the disease. Still, it is recognized that treatment with regular blood transfusions and iron chelation contributes to the etiology of bone loss.17
Imaging tests at this center (MRI and bone densitometry) are not performed in children under 8 years of age, and some children of age to allow us to perform densitometry have not yet done so.
Analyzing the results of abdominal ultrasound examinations, there was a discrepancy between the referred diseases and those recorded in the health records. Hepatosplenomegaly was reported by one patient, however on record there was splenomegaly. These results show the difficulties patients have in understanding the results of the complementary tests, due to the complexity of the disease and the procedures performed. The improvement in patient access to information and the manner in which communication and transmission of information pertinent to his or her state of health is accomplished, avoiding technical terms of difficult comprehension by the same, can be a manner to reverse this situation. Moreover, there are gaps related to clinical records in health records.
The limitations of this study refer to the sample size of Thal-no DM and Thal-DM patients for inferential statistical analysis, the age difference between the two groups, as well as the national studies comparing the results found in Thal-no DM and Thal-DM patients. It is known that in the Southeast region the services of care are allocated to patients with thalassemia major.4 However, future studies in partnership with other institutions are necessary for the advancement of knowledge on the subject. The patient's lack of knowledge about his or her comorbidities was also a limiting factor.
The complexity of the treatment of patients with TM requires that patients and healthcare professionals have specific knowledge to perform rigorous monitoring of therapy. Thus, knowing the sociodemographic and clinical characteristics of TM and TM patients with DM can provide tools for health professionals to understand the difficulties related to effective and safe care for this population.
ConclusionThese results allow us to conclude that the prevalence of diabetes mellitus was 16.1%. Patient ages ranged from 20 to 48 years, with an average of 35 years and most were students and those inserted in the formal job market. The most frequent treatment was the use of the oral chelator desferasirox and the interval of transfusional treatment was 15–22 days. Patients with thalassemia major and diabetes mellitus presented altered values of fasting glycemia, ALT, heart resonance and bone densitometry. Knowledge of the characteristics of this population favors reorientation on effective educational strategies due to the complexity of care and the progression of the disease.
Conflicts of interestThe authors declare no conflicts of interest.