The screening ofTrypanosoma cruzi-infected blood donors using two serological techniques frequently leads to conflicting results. This fact prompted us to evaluate the diagnostic performance of four “in-house” immunodiagnostic tests and two commercially available enzyme-linked immunosorbent assays (ELISAs).
Material and MethodsOne hundred and seventy-nine blood donors, whose screening for Chagas disease was doubtful, underwent three in-house ELISAs, one in-house immunoblotting test (TESA-blot), and two commercial ELISAs (bioMérieux and Wiener) in an attempt to define the presence or absence of infection. Simultaneously, 29 donors with previous positive results from three conventional serological tests and 30 donors with constant negative results were evaluated.
ResultsThe ELISA-Wiener showed the highest rate in sensitivity (98.92%) and the ELISA-bioMérieux, the highest specificity (99.45%), followed by the TESA-blot, which showed superior performance, with lower false-negative (2.18%) and false-positive (1.12%) rates. In series, the combination composed of the TESA-blot and ELISA-bioMérieux showed slightly superior performance, with trifunctional protein deficiency (TFP)=0.01%.
ConclusionOur study confirms the high sensitivity and specificity of commercial kits. To confirm the presence or absence of T. cruzi infection, the combination of TESA-blot and ELISA-bioMérieux may be suggested as the best alternative. Individually, the TESA-blot performed the closest to the gold standard; however, it is not commercially available.
The occurrence of inconclusive or indeterminate results in serological tests for Chagas disease in blood donor screenings, especially with the decrease in the prevalence of infection, is of increasing concern because they represent more than 50% of the non-negative serological reactions to the disease1–4. Several studies have demonstrated that the vast majority of these cases cannot be considered evidence of a Trypanosoma cruzi infection, suggesting the occurrence of false-positive reactions3,5.
In the 6-year period at the Uberaba Regional Blood Center (URBC), a total of 0.15% (140 samples) of the 95,990 blood donations collected were considered inconclusive for Chagas disease. This number (140 samples) represented 52% of the disabilities regarding the serology for T. cruzi, which was 0.29% in this period6. These data confirm the persistence of inconclusive results, which, in this study, occurred in both new and returning donors, including those with up to 29 repeatedly non-reactive donations. The search for confirmatory tests, such as the trypomastigote excreted-secreted antigen (TESA)-blot7,8,9, radioimmunoprecipitation assay10,11 and/or application of latent class analysis12, that can be routinely used in blood banks to define the exact profile of blood donors with 100% certainty has been the subject of several studies.
However, there are still difficulties regarding donors with inconclusive serological reactions, leading to the need to implement strategies to clarify, or at least minimize, ambiguous or inconclusive serological results7.
Thus, many researchers have improved the enzyme-linked immunosorbent assay (ELISA), which is the only required method for screening of blood donors at blood banks in Brazil8. This technique employs both antigens of the epimastigote or trypomastigote forms of T. cruzi as recombinant antigens, alone or in combination. Some of these are already available commercially or obtained "in-house," as in the case of the ELISA-recombinant complement regulatory protein (rCRP)9, or by using immunoglobulin subfractions.
In addition, IgG subclass analysis using immunofluorescence showed that the levels of immunoglobulin production were IgG1 > IgG3 > IgG2 > IgG4 in patients with chronic Chagas disease with cardiopathy12, which justifies the development of serological tests with different subclasses of IgG in ELISA.
Sensitivity, specificity and reagent standardization, as well as cross-reactivity, remain important issues to be resolved13 because, despite the evident decrease in the seroprevalence of Chagas disease in blood banks in most endemic countries, a significant number of refusals due to infection cannot be proved. In addition, inconclusive results can lead to additional social and psychological consequences for donors, who may be mistakenly labeled as having a serious illness, resulting in withdrawal from work and permanent inability to donate blood. These facts justify our search for alternatives to ELISA in to minimize or exclude the occurrence of inconclusive results.
MethodsStudy populationSerum samples from 238 individuals were analyzed and classified into three groups: True-positives: 29 blood donors from the Uberaba Regional Blood Center with repeatedly positive results in three different conventional serological (CS) tests [ELISA, indirect hemagglutination (IHA) and indirect immunofluorescence], with 40% testing positive in a hemoculture test; Tue-negatives: 30 blood donors from the Uberaba Regional Blood Center with more than five donations and repeatedly negative serology in three different CS tests, and; inconclusive donors: 179 blood donors at the blood bank of Santa Casa de São Paulo who presented inconclusive screening results, with two conflicting CS results (ELISA and IHA) or ELISA values within the gray area (± 20% of the cutoff). All samples were obtained from the serum bank of the respective services, collected between 2012 and 2014, where they were kept at -30°C and processed in duplicate.
Tests for Chagas diseaseTwo commercial ELISA kits were evaluated, one from bioMérieux (Chagatek, Brazil), which uses the epimastigote forms of T. cruzi as an antigen, and another from Wiener (recombinant Chagatest v3.0 Lab, Rosario, Argentina), which uses a cocktail of recombinant proteins. Both kits were processed according to the manufacturer’s recommendations.
We also evaluated four “in-house” tests as described below:
ELISA-rCRPA recombinant protein purified, as described by Meira et al. (2002), and obtained after the purification of a recombinant trypomastigote-specific surface glycoprotein (complement regulatory protein) was used for the coating of ELISA plates, according to the described technique, with some modifications. Flat-bottom ELISA plates (Hybound, Costar, USA) were coated with recombinant Tc-PCR (2.5mg/mL) at 4°C for 18h. Plates were blocked with PBS containing 5% skim milk (Molico Nestlé Brazil Ltda., Araçatuba, SP, Brazil) for 2h at 37°C. After blocking, 50μL (1:200) of sera was added and incubated for 1h at 37°C. After additional washes with PBS supplemented with 0.05% Tween 20 (PBS-T), 50μL of conjugate anti-human IgG linked to peroxidase was added (peroxidase-conjugated ty67 Rabbit Anti-Human IgG, DAKO A/S Glostrup, Denmark) at 1:7500 and incubated for 1h at 37° C. The antigen–antibody complex was added to 100mL of 3,3',5,5'-tetramethylbenzidine (TMB; DAKO Corp. Carpinteria, CA, USA) for 1h at 37°C in the dark. After exposure to 1N H2SO4, the plates were read at 450nm (Benchmark Microplate Reader, BIO-RAD, Hercules, CA, USA). In all ELISAs, eight serum samples from chronic chagasic patients were used as positive controls and eight from healthy donors were used as negative controls. The cutoff, determined individually on each plate, was calculated as the average of the negative controls plus two times the standard deviation of these samples.
ELISA-IgG1 and ELISA-IgG3The two ELISAs were performed using the commercial ELISA-Wiener kit (recombinant Chagatest v3.0 Lab, Rosario, Argentina) with specific immunoglobulin subclasses. For the testing of IgG1 and IgG3 subclasses, donor samples were incubated for 2h in commercial plates from Wiener and rinsed as per the manufacturer's instructions. Subsequently, a new incubation was performed with the respective immunoglobulins IgG1 and IgG3 (Anti-human; Sigma-Aldrich, St Louis, MO, USA) diluted 1:500 (1% BSA-PBS) for 2h at 37°C. After this incubation period with IgG1 and IgG3, the protocol was followed by four successive washes of the plates and incubation for 1h with streptavidin peroxidase (Sigma, Co) diluted 1:1000 in PBS-BSA 2%. The plates were then washed four times and tested using the Wiener substrate kit, according to the manufacturer's instructions.
The absorbance was measured using a microplate reader (BioRad 3550 Microplate Reader) with a 405-nm filter. The results were expressed as absorbance.
Immunoblotting: TESA-blotThe T. cruzi Y strain for the TESA-blot was obtained, as previously described (Umezawa, et al., 1996): TESA fractions, electrophoretically separated on 7% SDS-PAGE gel, was blotted onto nitrocellulose membranes (0.45μm). Membrane strips (5mm) blocked with PBS containing 5% fat-free milk (Molico, Nestlé) were incubated with serum samples (1:200) diluted in PBS containing 1% milk for 2h or overnight at room temperature. The immunocomplexes were revealed after 2 more hours of incubation with anti-human IgG-peroxidase (Sigma Co) with the addition of 0.05% hydrogen peroxide and 4-chloro-1-naphthol, as described by Umezawa et al. (1996). Samples were considered positive when a large, 150–160kDa band, and/or five bands between 130 and 200kDa were observed.
Data analysisWith prior information, a Bayesian latent class model was used to estimate the performance parameters. Dependence structure of Se and Sp and positive (PPV) and negative (NPV) predictive values for diagnostic tests under investigation, as well as prevalence rates of T. cruzi infection among the inconclusive serological screening group, were considered and the population was stratified based on assumptions of Hui and Walter14. The real, but unknown, donor health status (uninfected or infected) was determined using the Bernoulli distribution probability. Besides the individual performance, we also estimated the performance of pairs of tests in parallel and in series. In each of the groups (negative control, positive control, and inconclusive), the test results within the gray area (inconclusive) were included in the analysis of test performance and were considered positive if at least three other tests showed positive results. The ELISA-rCRP and ELISA-IgG3 tests were excluded from the analysis due to their low performance.
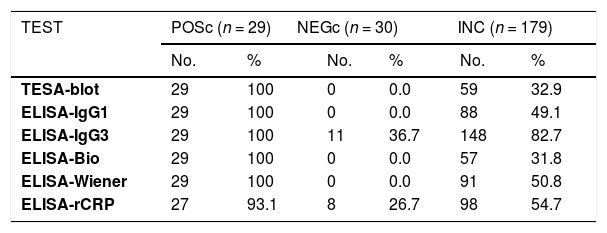
ResultsThe ELISA-bioMérieux and ELISA-Wiener kits and ELISA-IgG1, ELISA-IgG3, and TESA-blot provided 100% positivity when they were evaluated with samples from the true-positive control group (n=29), with the exception of ELISA-rCRP, that showed a positivity of 93.1%. The ELISA-bioMérieux, ELISA-Wiener, ELISA-IgG1, as well as the TESA-blot showed no positive cases (0%) when samples from the true-negative control group (n=30) were tested, but different positivities were obtained for the two in-house ELISAs (26.7% for rCRP and 36.7% for IgG3). Among the 179 donors in the inconclusive group, similar positivities were reached using the ELISA-bioMérieux (31.8%) and TESA-blot (32.9%) tests. Different positivities were obtained using the ELISA-IgG1 (49.1%), ELISA-Wiener (50.8%), and ELISA-rCRP (54.7%) tests and an unexpectedly high positivity was obtained using the ELISA-IgG3 (82.7%) (Table 1).
Positivity in Chagas disease diagnostic tests in true chagasic (positive control), true non-chagasic (negative control), and inconclusive cases.
| TEST | POSc (n = 29) | NEGc (n = 30) | INC (n = 179) | |||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| TESA-blot | 29 | 100 | 0 | 0.0 | 59 | 32.9 | ||
| ELISA-IgG1 | 29 | 100 | 0 | 0.0 | 88 | 49.1 | ||
| ELISA-IgG3 | 29 | 100 | 11 | 36.7 | 148 | 82.7 | ||
| ELISA-Bio | 29 | 100 | 0 | 0.0 | 57 | 31.8 | ||
| ELISA-Wiener | 29 | 100 | 0 | 0.0 | 91 | 50.8 | ||
| ELISA-rCRP | 27 | 93.1 | 8 | 26.7 | 98 | 54.7 | ||
POSc: positive control, NEGc: negative control, Inc: inconclusive, TESA-blot: trypomastigote excreted-secreted antigens, ELISA-IgG1 e IgG3: ELISA IgG imunoglobulins subfractions IgG1, IgG3 e IgG T: ELISA-Bio: ELISA-bioMériéux, ELISA-Wiener: ELISA-Wiener, ELISA-rCRP: ELISA-recombinant complement regulatory protein.
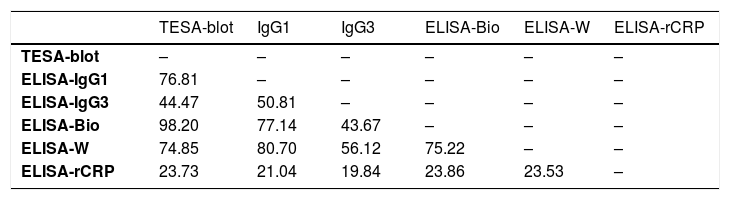
We observed, using the Kendall’s coefficient, higher levels of agreement (≥ 70%) among the following tests under investigation: TESA-blot with ELISA-bioMérieux and IgG1e ELISA-Wiener (98.20%, 76.81%, and 74.85%, respectively), IgG1-ELISA with the ELISA-Wiener-bioMérieux (80.70% and 77.14%, respectively) and the ELISA-bioMerieux with ELISA-Wiener (75.22%). Due to their low performance, ELISA and ELISA-RCRP-IgG3 tests were excluded from the analysis. (Table 2)
Kendall’s coefficient of concordance (%) between the pairs of tests.
| TESA-blot | IgG1 | IgG3 | ELISA-Bio | ELISA-W | ELISA-rCRP | |
|---|---|---|---|---|---|---|
| TESA-blot | – | – | – | – | – | – |
| ELISA-IgG1 | 76.81 | – | – | – | – | – |
| ELISA-IgG3 | 44.47 | 50.81 | – | – | – | – |
| ELISA-Bio | 98.20 | 77.14 | 43.67 | – | – | – |
| ELISA-W | 74.85 | 80.70 | 56.12 | 75.22 | – | – |
| ELISA-rCRP | 23.73 | 21.04 | 19.84 | 23.86 | 23.53 | – |
T-Blot: trypomastigote excreted-secreted antigens, ELISA-IgG1 e IgG3: ELISA-IgG imunoglobulins subfractions IgG1, IgG3 e IgGT: E-Bio: ELISA-bioMériéux, E-Wiener: ELISA-Wiener, rCRP: recombinant complement regulatory protein.
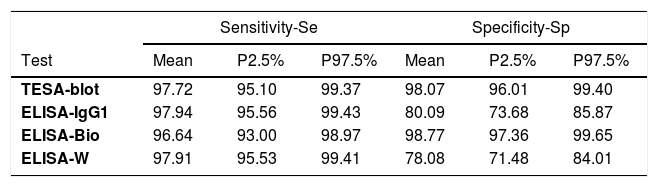
Performance parameters of each of the four tests showed that the ELISA-IgG1had the highest sensitivity (Se=97.94%), followed by the ELISA-Wiener (97.91%), TESA-blot (97.72%) and ELISA-bioMérieux (96.64%), whereas the highest specificity (Sp) was observed for the ELISA-bioMérieux (98.77%), followed by the TESA-blot (98.07%) and ELISA-Wiener (78.08%) (Table 3). The best performance test is the one that has the lowest false-positive (FPR=1-Sp) and false-negative (FNR=1-Se) rates. Among the four diagnostic tests under investigation, the TESA-blot showed the best performance, with the lowest FNR (2.28%) and FPR (1.93%), followed by ELISA-bioMérieux (FNR=3.36%, FPR=1.23%). Although the ELISA-IgG1 and ELISA-Wiener showed good performance for diagnosing the presence of T. cruzi infections (FNR=2.06% and FNR=2.09%, respectively), they were ineffective in detecting the absence of Chagas disease (FPR=26.32%, FPR=21.92%, respectively) (Table 3).
Sensitivity and specificity of the individual tests.
| Sensitivity-Se | Specificity-Sp | |||||
|---|---|---|---|---|---|---|
| Test | Mean | P2.5% | P97.5% | Mean | P2.5% | P97.5% |
| TESA-blot | 97.72 | 95.10 | 99.37 | 98.07 | 96.01 | 99.40 |
| ELISA-IgG1 | 97.94 | 95.56 | 99.43 | 80.09 | 73.68 | 85.87 |
| ELISA-Bio | 96.64 | 93.00 | 98.97 | 98.77 | 97.36 | 99.65 |
| ELISA-W | 97.91 | 95.53 | 99.41 | 78.08 | 71.48 | 84.01 |
P2.5% and P97.5%: percentile 2.5% and 97.5%, respectively, FPR: false-positive rate (1-Sp), FNR: false-negative rate (1-Se), TESA-blot: trypomastigote excreted-secreted antigens, ELISA-IgG1 and IgG3: ELISA IgG immunoglobulins subfractions IgG1 and IgG3, ELISA-Bio: ELISA-bioMériéux, ELISA-W: ELISA-Wiener, DIC: Deviance Information Criterium.
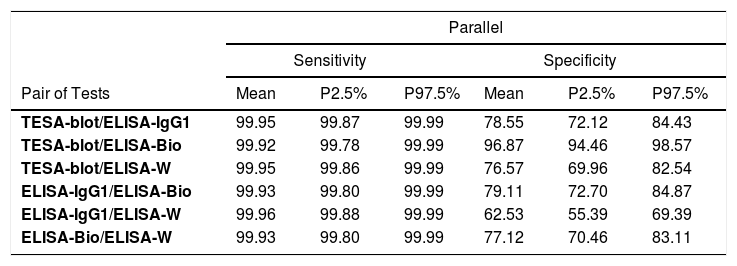
The four tests (TESA-blot, ELISA-IgG1, ELISA-bioMérieux, and ELISA-Wiener) were used in pairs, according to the parallel scheme, to show the most appropriate screening for Chagas disease, with an FNR very close to 0%. As for the series scheme, which is suitable for disease confirmation, the TESA-blot and ELISA-bioMérieux pair (FPR=0.02%) presented a slightly better performance, followed by the ELISA-IgG1 and ELISA-bioMérieux and ELISA-bioMérieux and ELISA-Wiener (FPR=0.24%, FPR=0.27%, respectively) (Table 4).
Sensitivity and specificity of the pair of tests using parallel and series schemes.
| Parallel | |||||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | ||||||
| Pair of Tests | Mean | P2.5% | P97.5% | Mean | P2.5% | P97.5% | |
| TESA-blot/ELISA-IgG1 | 99.95 | 99.87 | 99.99 | 78.55 | 72.12 | 84.43 | |
| TESA-blot/ELISA-Bio | 99.92 | 99.78 | 99.99 | 96.87 | 94.46 | 98.57 | |
| TESA-blot/ELISA-W | 99.95 | 99.86 | 99.99 | 76.57 | 69.96 | 82.54 | |
| ELISA-IgG1/ELISA-Bio | 99.93 | 99.80 | 99.99 | 79.11 | 72.70 | 84.87 | |
| ELISA-IgG1/ELISA-W | 99.96 | 99.88 | 99.99 | 62.53 | 55.39 | 69.39 | |
| ELISA-Bio/ELISA-W | 99.93 | 99.80 | 99.99 | 77.12 | 70.46 | 83.11 | |
| Series | |||||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | ||||||
| Pair of Tests | Mean | P2.5% | P97.5% | Mean | P2.5% | P97.5% | |
| TESA-blot/ELISA-IgG1 | 95.71 | 92.42 | 98.11 | 99.62 | 99.17 | 99.89 | |
| TESA-blot/ELISA-Bio | 94.44 | 90.26 | 97.48 | 99.98 | 99.93 | 100.00 | |
| TESA-blot/ELISA-W | 95.68 | 92.28 | 98.07 | 99.58 | 99.09 | 99.87 | |
| ELISA-IgG1/ELISA-Bio | 94.65 | 90.60 | 97.58 | 99.76 | 99.45 | 99.94 | |
| ELISA-IgG1/ELISA-W | 95.89 | 92.82 | 98.19 | 95.64 | 93.56 | 97.27 | |
| ELISA-Bio/ELISA-W | 94.62 | 90.49 | 97.60 | 99.73 | 99.40 | 99.93 | |
P2.5% and P97.5%: percentile 2.5% and 97.5%. respectively, FPR: false-positive rate (1-Sp), FNR: false-negative rate (1-Se), TESA-blot: trypomastigote excreted-secreted antigens, ELISA-IgG1 and IgG3: ELISA IgG imunoglobulins subfractions IgG1. IgG3 and IgG T: ELISA-Bio: ELISA-bioMériéux, ELISA-W: ELISA-Wiener, DIC: Deviance Information Criterium.
We estimated the positive results for Chagas disease in 33.63% of the 179 donors with inconclusive serology screenings.
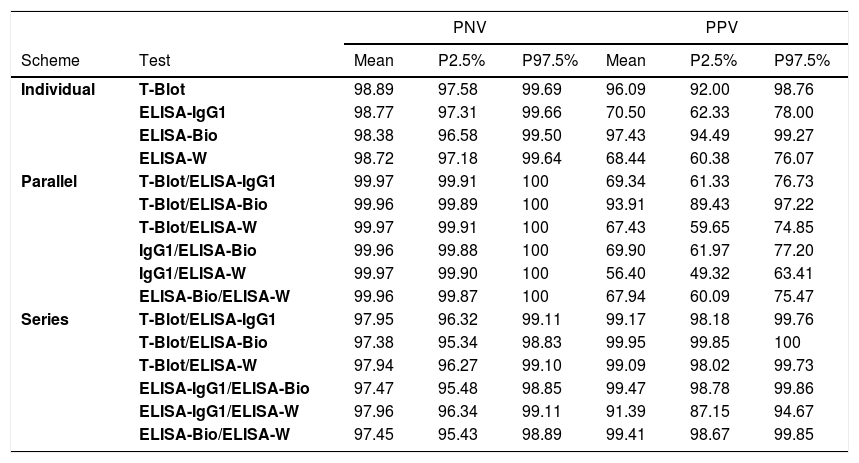
The best performance (ability) of the individual tests to predict the presence (PPV) or absence (NPV) of a T. cruzi infection in the serological screenings of the inconclusive group was observed using the ELISA-bioMérieux (PPV=98.38% and PNV=97.43%). More clearly stated, among the blood donors with inconclusive results, when the test result was positive, the probability of T. cruzi infection was 98.38% (PPV), and when the test result was negative, the likelihood of the absence of Chagas disease was 97.43% (PNV) (Table 5).
Negative and positive predictive values from the individual, parallel and series schemes of the inconclusive screening results group.
| PNV | PPV | ||||||
|---|---|---|---|---|---|---|---|
| Scheme | Test | Mean | P2.5% | P97.5% | Mean | P2.5% | P97.5% |
| Individual | T-Blot | 98.89 | 97.58 | 99.69 | 96.09 | 92.00 | 98.76 |
| ELISA-IgG1 | 98.77 | 97.31 | 99.66 | 70.50 | 62.33 | 78.00 | |
| ELISA-Bio | 98.38 | 96.58 | 99.50 | 97.43 | 94.49 | 99.27 | |
| ELISA-W | 98.72 | 97.18 | 99.64 | 68.44 | 60.38 | 76.07 | |
| Parallel | T-Blot/ELISA-IgG1 | 99.97 | 99.91 | 100 | 69.34 | 61.33 | 76.73 |
| T-Blot/ELISA-Bio | 99.96 | 99.89 | 100 | 93.91 | 89.43 | 97.22 | |
| T-Blot/ELISA-W | 99.97 | 99.91 | 100 | 67.43 | 59.65 | 74.85 | |
| IgG1/ELISA-Bio | 99.96 | 99.88 | 100 | 69.90 | 61.97 | 77.20 | |
| IgG1/ELISA-W | 99.97 | 99.90 | 100 | 56.40 | 49.32 | 63.41 | |
| ELISA-Bio/ELISA-W | 99.96 | 99.87 | 100 | 67.94 | 60.09 | 75.47 | |
| Series | T-Blot/ELISA-IgG1 | 97.95 | 96.32 | 99.11 | 99.17 | 98.18 | 99.76 |
| T-Blot/ELISA-Bio | 97.38 | 95.34 | 98.83 | 99.95 | 99.85 | 100 | |
| T-Blot/ELISA-W | 97.94 | 96.27 | 99.10 | 99.09 | 98.02 | 99.73 | |
| ELISA-IgG1/ELISA-Bio | 97.47 | 95.48 | 98.85 | 99.47 | 98.78 | 99.86 | |
| ELISA-IgG1/ELISA-W | 97.96 | 96.34 | 99.11 | 91.39 | 87.15 | 94.67 | |
| ELISA-Bio/ELISA-W | 97.45 | 95.43 | 98.89 | 99.41 | 98.67 | 99.85 | |
PNV: predictive negative value, PPV: predictive positive value, TESA-blot: trypomastigote excreted-secreted antigens, ELISA-IgG1 and IgG3: ELISA IgG imunoglobulins subfractions IgG1. IgG3 and IgG T: ELISA-Bio: ELISA-bioMériéux, ELISA-Wiener: ELISA-Wiener.
Considering a parallel scheme, when the group tested using the TESA-blot and ELISA-bioMérieux presented positive results from at least one test, there was a 93.91% probability of predicting the presence (PPV) of T. cruzi infection in donors with inconclusive serology. In the same way, when the group tested using the TESA-blot and ELISA-bioMérieux presented positive results from both tests, there was a 99.95% probability of predicting the presence of T. cruzi infection in donors with previously inconclusive serology. Furthermore, when at least one of the tests (ELISA or ELISA-IgG1-Wiener) provided negative results in the series scheme, the likelihood of predicting the absence of T. cruzi infection was 97.96% for donors with inconclusive serology (Table 5).
DiscussionThe rate of Chagas disease in blood donors has been considerably reduced in the last decade. Between 2012 and 2014, according to data from the respective statistical bulletins, the serological disability index for Chagas disease at the URBC was only 0.16% and at the blood bank of Santa Casa de São Paulo it was 0.17%. However, among those deemed unfit to donate, a significant percentage, up to 80% of non-negative results, are serologically inconclusive3,4,6,20. Many studies still report that these inconclusive samples, after being analyzed using various other tests, showed repeated negative results. In our study, we found that 66.88% of the inconclusive serological screening results were negative for Chagas disease, according to the TESA-blot test, which is considered highly sensitive and specific15. Similar results were found in other studies3,7.
When evaluating commercial kits, the ELISA-bioMérieux showed the highest specificity (99.45%) and the ELISA-Wiener showed the highest sensitivity (98.92%). This study agrees with other studies that reported the sensitivity for the Wiener recombinant kit varying from 98% to 100% and specificity from 95% to 99%16,17. For the ELISA-bioMérieux, which uses the crude extract of the parasite lysate, a sensitivity of 100% has been reported; however, this kit may have levels of specificity ranging from 82% to 98.5%18,19.
When evaluating the combination of the ELISA-IgG with other tests, the IgG1/ELISA-Wiener tests showed a low rate of FNR (0.01%), according to the parallel scheme, and the IgG1/BioMérieux tests showed a low rate of FPR (0.09%), according to the series scheme, suggesting that the ELISA-IgG1 in conjunction with the ELISA-bioMérieux could be used in blood banks for the confirmation of Chagas disease.
The main causes of failure of the commercial ELISA kits for Chagas disease are the cross-reactivity with other parasites, especially with the genus Leishmania sp, which is also a trypanosomatide that shares many genetic similarities with T. cruzi and its antigens and may present the same epitopes for binding to antibodies in the serum of infected patients20. However, in a previous study that evaluated the cross-reactivity between the two trypanosomiases in donors who tested positive for Chagas disease, we confirmed the high specificity of serological tests used in regions endemic to the two trypanosomiases21. Similar results were also obtained in the present study. We obtained sensitivity rates of 99.99% when analyzing the IgG1/Wiener-ELISA set according to the parallel scheme and specificity of 99.99% for the TESA-blot/ELISA-bioMérieux when analyzed in series. However, it was not possible to establish a diagnostic test that could individually confirm chronic Chagas disease.
In general, our study confirms the high sensitivity and specificity of commercial kits produced by bioMérieux and Wiener, which may remain as the serological screening techniques of choice at blood banks22. The combination that presented the lowest FPRs according to the series scheme and one of the FNRs according to the parallel scheme was the TESA-blot and ELISA-bioMérieux set. When both are positive, they may be suggested as the best alternatives to confirm the presence of the T. cruzi infection, and when negative, they demonstrate the absence of infection, especially in cases of previous inconclusive serology.
Individually, the TESA-blot test performed the closest to the gold standard with the lowest FPRs and FNRs and could be used to confirm inconclusive results at blood banks, as already mentioned in the literature10,12. Unfortunately, it is not currently available. Given this and the high sensitivity and facilities for automation, the ELISA-Wiener was confirmed in this study as being the most suitable single screening test for Chagas disease in blood donors.
In addition, studies have suggested the application of a clinical-epidemiological questionnaire as a method to aid in donor screening, which may help in accepting or excluding donors at risk for Chagas disease3,6,22· In a previous study, we observed that the chance for T. cruzi seropositivity was four times higher for individuals living in rural endemic regions, eleven times greater for those who reported having been in contact with triatomine, and 4.8 times higher for those who reported cases of Chagas disease in their close relatives6. The use of this questionnaire has been the first choice for donor selection in some non-endemic countries in Europe where migration is more intense, such as France, Italy, Spain, and the UK 3,21,22.
Thus, in addition to increasing the safety of blood transfusions, the combination of high performance tests, such as the TESA-blot and ELISA-BioMérieux/Wiener and epidemiological data may provide a more accurate view of the serological profile of blood donors. Such measures can significantly contribute to reducing the unnecessary disposal of blood bags and the number of donors erroneously classified as having a T. cruzi infection.
Finnancial supportAgência Nacional de Vigilância Sanitária – ANVISA.
Conflicts of interestThe authors declare no conflicts of interest
Thanks to the blood donors and employees of the Hemominas Foundation, of the Federal University of Triângulo Mineiro and IMT-FMUSP, for their assistance with the study. Thanks to the Agência Nacional de Vigilância Sanitária (ANVISA) and CAPES.