Coronavirus disease 2019 (COVID-19) may present with extrapulmonary manifestations, including hematologic changes. Previous studies suggest that severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) can interact with the renin-angiotensin system, ultimately causing increased production of angiotensin II. By reporting the cases of previously healthy young adults diagnosed with a hematologic malignancy after experiencing COVID-19, we raise the hypothesis that the SARS-Cov-2 infection could act as a trigger for leukemogenesis in predisposed individuals.
MethodsThis was a case series performed through extraction of relevant clinical information from the medical records of three patients admitted to our Hematology unit between August 2020 and September 2020.
Main ResultsConsidering the relatively rapid development of cytopenias following recovery from COVID-19, it cannot be ruled out that SARS-Cov-2 played a role in leukemogenesis in those patients. Based on previous in vitro studies, the renin-angiotensin system imbalance induced by SARS-CoV-2 could potentially promote in vivo leukemogenesis through several mechanisms.
ConclusionDespite the advances in pathophysiological and clinical characterization of COVID-19, the consequences of the pandemic to the incidence of hematologic diseases are still to be elucidated. In this context, future dissection of the status of the local bone marrow renin-angiotensin system in leukemogenesis is a clinically relevant basic research area.
Since December 2019, when its first cases were recognized in Wuhan, China, the Coronavirus disease 2019 (COVID-19) has rapidly spread through more than 200 countries.1 Although severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) is most well-known for causing mild to significant pulmonary symptoms, it may also induce several extrapulmonary manifestations, including hematologic changes.2-4
Abnormal immune response to viral infections can indirectly trigger the secondary mutational events that promote clinical leukemia development.5 Additionally, SARS-Cov-2 can interact significantly with the renin-angiotensin system (RAS), which has been suggested to have a role in neoplastic hematopoiesis.6,7 Thus, we hypothesize that SARS-Cov-2 could potentially act as the second-hit needed for leukemogenesis, inducing hematologic neoplasia in genetically predisposed individuals.
Considering the above, the present study aims to report the cases of three previously healthy young adults who were diagnosed with a hematologic malignancy a few months after an acute presentation of COVID-19.
MethodsThis case series was performed through extraction of relevant clinical information from the medical records of three patients admitted between August 2020 and September 2020 to the Walter Cantídio University Hospital (HUWC) of the Federal University of Ceará School of Medicine (FAMED-UFC), Fortaleza, Brazil. The three patients gave us verbal and written consent for description of their respective cases. All personal identifiers have been removed. Finally, in the attempt to explain the phenomenon, review of the literature published during the last 20 years regarding the effects of the RAS on the bone marrow (BM) was performed using the PubMed/MEDLINE database.
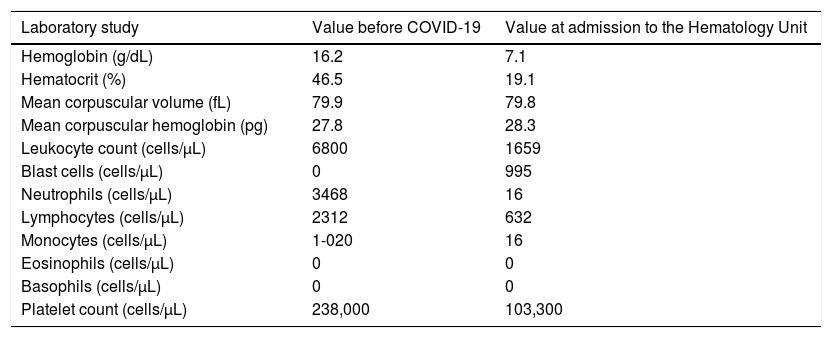
Case seriesCase 1A 35-year-old male was referred to our service due to moderate fatigue and complete blood count (CBC) showing pancytopenia and lymphocytosis. The patient had no signs of bleeding, splenomegaly, lymphadenopathy or extranodal involvement. Although he had no comorbidities, his past medical history was significant for COVID-19, with a positive Nutriex® colloidal gold-based immunochromatographic strip assay for IgM against SARS-CoV-2 on July 7, 2020. He performed the test after experiencing fever, chills, myalgias, mild dyspnea and anosmia for 3 days. At that time, he was managed as an outpatient, with full remission of symptoms after 10 days. His baseline CBC before COVID-19 symptoms was normal, as indicated in Table 1.
A 35-year-old male was diagnosed with T-cell acute lymphoblastic leukemia (T-ALL) approximately 2 months after experiencing COVID-19. Results of his complete blood count with the differential before SARS-CoV-2 infection and at admission to the Hematology Unit are indicated.
Peripheral blood flow cytometry was performed, revealing 77% of small blast cells, with a strong expression of cluster of differentiation (CD) 34, cytoplasmic CD3, CD8, CD7 and CD10; weak expression of CD19 and CD13, and; negativity for CD45, CD2, CD4, CD5, CD1a, CD13, CD33, CD33, CD117 and CD79a. Thus, the immunophenotypic profile of the patient sample was compatible with the diagnosis of T-cell acute lymphoblastic leukemia (ALL-T). Real-time reverse transcriptase - polymerase chain reaction (RT-PCR) for SARS-CoV-2 detection on a nasopharyngeal sample was negative on September 3, 2020.
He was admitted to the Hematology Unit on September 7, 2020 for the management of neutropenic fever and further diagnostic evaluation. The myelogram revealed 90% of blast cells, characterized by small size, high nucleus-to-cytoplasm ratio, evident nucleus and scarce basophilic cytoplasm, with no granulations. Hypoplasia of erythroid lineage (3% of the cells) and granulocytic lineage (7% of the cells) was present and there was no visualization of megakaryocytes. Additionally, the cerebrospinal fluid analysis revealed 21% of blast cells, suggesting leukemic involvement of the central nervous system. On September 14, 2020, induction chemotherapy for acute lymphocytic leukemia (ALL) with the Cancer and Leukemia Group B Study 9511 (CALGB 9511) protocol was initiated.8
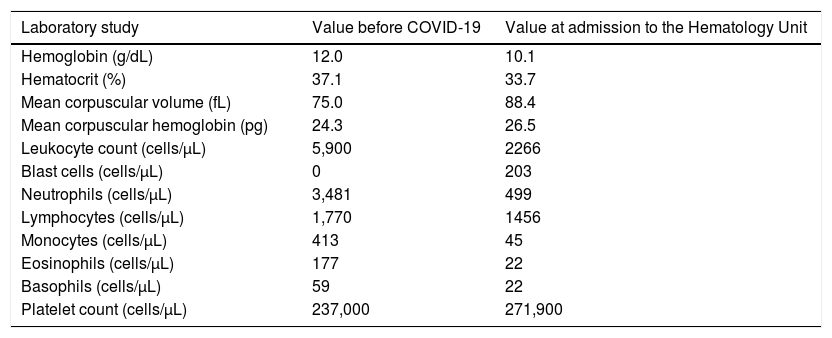
Case 2A 36-year-old male was referred to our service due to normocytic anemia, neutropenia and the presence of blast cells in the peripheral blood smear. His family history was significant for parental consanguinity and acute myeloid leukemia (AML) in one sister and four first cousins. Besides chronic active gastritis due to untreated Helicobacter pylori infection, his past medical history revealed a 3-day hospitalization for presumed COVID-19 one month earlier. His baseline CBC showed only mild microcytic, hypochromic anemia, as indicated in Table 2. After improvement in the respiratory symptoms, he was discharged, but persisted with dry cough and fever. On August 18, 2020, the SARS-CoV-2 infection was confirmed by the RT-PCR of a nasopharyngeal specimen.
A 36-year-old male was diagnosed with myelodysplastic syndrome with excess blasts type 1 (MDS-EB-1) approximately 2 months after experiencing COVID-19. Results of his complete blood count with the differential before SARS-CoV-2 infection and at admission to the Hematology Unit are indicated.
On August 24, 2020, the patient was admitted to the Hematology Unit to proceed with diagnostic investigation. The flow cytometry on the bone marrow (BM) aspirate revealed 9.25% of myeloid blast cells, with the strong expression of CD117; moderate expression of MPO, CD34 and CD45; weak expression of CD33 and CD13, and; negativity for CD79a, CD10, CD19, CD11b/c, CD7, CD64, CD56 and CD14. The histopathological analysis following the BM biopsy showed bone trabeculae permeated by hematopoietic matrix with 95% cellularity and grade 1 myelofibrosis (MF-1). Elements of the three lineages were present, with frequent immature forms of intertrabecular localization. The immunohistochemistry (IHC) confirmed hypercellular BM for age with 8% of myeloid blasts (positive staining for CD34 and CD117), in association with microvascular proliferation, 10% of proerythroblasts and 30% of micromegakaryocytes. No Auer rods were detected in the peripheral blood or BM blasts. The fluorescence in situ hybridization (FISH) was negative for the deletion of long arm of chromosome 5 (5q-). However, 100% of the cells in the analyzed sample were positive for monosomy/deletion of the long arm of chromosome 7q (−7/7q−). The FLT3 and NPM1 mutation analyses in the peripheral blood were negative.
Finally, the diagnosis of myelodysplastic syndrome with excess blasts type 1 (MDS-EB-1) was made.9 The collection of blood samples for human leukocyte antigens (HLA) typing was performed, foreseeing the possibility of future allogeneic hematopoietic cell transplantation (HCT). On September 19, 2020, the patient was discharged from the Hematology Unit. He currently awaits the results of HLA typing of potential donors.
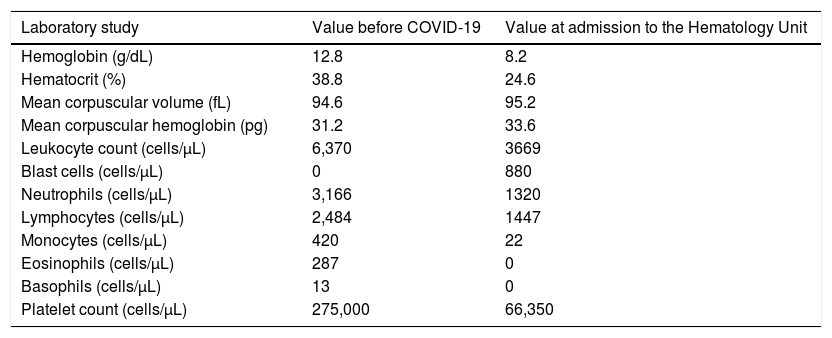
Case 3On September 19, 2020, a 31-year-old female with a 1-month history of asthenia, fatigue, petechiae on dependent areas and bone pain in the lower limbs and thoracic spine was admitted to the Hematology Unit for diagnostic investigation. There were no signs of active bleeding, splenomegaly or lymphadenopathy. At the time of admission, her CBC revealed pancytopenia and blast cells in the peripheral blood. However, her baseline CBC performed 5 months before admission was normal, as indicated in Table 3.
A 31-year-old female was diagnosed with acute myeloid leukemia (AML) approximately 3 months after experiencing COVID-19. Results of her complete blood count with the differential before the SARS-CoV-2 infection and at admission to the Hematology Unit are indicated.
Approximately 3 months before hospitalization, she experienced mild symptoms of COVID-19 for 2 weeks, including increased mucus production, anosmia, ageusia and headache. On September 9, 2020, RT-PCR, in a nasopharyngeal sample, she still tested positive for the SARS-CoV-2 infection. A new test performed 8 days later yielded a negative result.
A myelogram performed during hospitalization revealed an intensely hypercellular BM, with dysplasia in all three lineages. Furthermore, 22% of blast cells, characterized by a slightly basophilic cytoplasm with granulations, were present. The flow cytometry on the BM aspirate revealed 19% of myeloid blast cells, with a strong expression of the human leukocyte antigen – DR isotype (HLA-DR), CD34, MPO, CD117, CD13 and CD33; moderate expression of CD45, and; negativity for CD10, CD19, CD11b/c, CD3, CD7, CD14, CD15, CD16 and CD64. The qualitative RT-PCR for the detection of BCR-ABL transcripts was negative, as well as the FLT3 and NPM1 mutation analyses in the peripheral blood.
The diagnosis of AML, probably secondary to the previous MDS, was made. On September 22, 2020, the “7+3 therapy” with cytarabine plus idarubicin was initiated for remission induction.10 Finally, due to plans for future allogeneic HCT, we collected blood samples from the patient and potential donors for the HLA typing.
DiscussionCOVID-19 has been associated with multiple short- and long-term cardiopulmonary, neuropsychiatric, endocrinologic, renometabolic, hematologic, ophthalmologic, dermatologic, gastrointestinal and hepatobiliary complications.2 Despite the recent progress in elucidating its pathophysiological processes, many aspects remain unclear and continue to impose a challenge to the scientific community.4
Emerging literature suggests that the SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) via its spike protein,11 allowing the virus to invade cells in different tissues, including the BM.12 This causes the downregulation of ACE2, either directly due to viral binding or indirectly due to cell lysis.13 Physiologically, the ACE2 provides a counterregulatory mechanism of angiotensin II (Ang-II) production by the ACE. Thus, the SARS-CoV-2 infection can enhance Ang-II signaling via the Ang-II receptor type 1 (AT1R), leading to immune cell activation and lung injury.14
Through autocrine and paracrine mechanisms, the RAS can contribute to the regulation of normal and malignant hematologic processes in the BM.15 Local expression of angiotensinogen, renin, ACE, Ang-II, AT1R and AT2R can be detected in both hematopoietic and stromal compartments, while the expression of ACE2 is present only in the non-stromal compartment.16 Abnormalities in the BM microenvironment due to dysfunctional local RAS may be one of the main mechanisms underlying COVID-19-induced hematologic findings (e.g., lymphopenia, neutrophilia, thrombocytopenia and abnormalities in coagulation parameters).3,11
Although several studies report the clinical characteristics and management challenges of patients with concurrent COVID-19 and malignant hematologic disorders,17-21 this is the first study to hypothesize a possible causal relationship between COVID-19 and hematologic neoplasia. Despite the likelihood that the association is only coincidental, especially considering the pandemic context,1 a review of the current literature disclosed a plausible theoretical mechanism by which the SARS-CoV-2 infection is linked with the subsequent development of hematologic malignancy in predisposed patients.
According to the 2-hit hypothesis, most acute leukemias result from the collaboration of multiple consecutive genetic changes.22 Initially, a hematopoietic stem cell in the BM acquires a competitive advantage through determined somatic mutations, giving rise to an expanded clone of leukocytes that circulate in peripheral blood, a condition now called clonal hematopoiesis of indeterminate potential (CHIP).23 The subsequent progression from CHIP to leukemia, which occurs in a minority of patients, usually requires secondary mutational events.22,23
Recent data indicate that CHIP is associated with a significant increase in cardiovascular risk that is independent on well-recognized risk factors.24 While the causes for such an association are still under investigation,23 cumulative laboratory evidence suggests that an exaggerated activation of the classical arm of the RAS (i.e., ACE/Ang-II/AT1R), which has been consistently implicated in the pathogenesis of many cardiovascular conditions, may promote neoplastic hematopoiesis through several mechanisms.7 Correspondingly, renin, angiotensinogen, ACE and Ang-II have already been identified in leukemic blast cells.25
Based on previous in vitro studies, the RAS imbalance induced by SARS-CoV-2 could potentially promote in vivo leukemogenesis.7 In cultured renal cells, the administration of Ang-II led to DNA damage, including single- and double-strand breaks.26 In addition to evidence that its increased production could result in the leukemic transformation of BM hematopoietic cells,27 Ang-II has been suggested to act as an autocrine growth factor for AML cells.28 It has induced the proliferation of CD34+ hematopoietic progenitor cells isolated from murine BM and human cord blood, an effect which was blocked by losartan, indicating mediation via the AT1R.29 Downstream AT1R signaling can additionally improve pro-survival anti-apoptotic signaling in neoplastic cells.7 This was demonstrated by the induction of apoptosis in leukemic myeloid cell lines treated with captopril, trandolapril or losartan30 and of apoptosis in adult T-cell leukemia cells treated with telmisartan31 and enhancement of the antineoplastic effect of doxorubicin after combination with losartan.32
In addition to the influence of SARS‐CoV‐2 on the RAS, other mechanisms could underlie its potential to hasten cancer development.33,34 In a 2012 study, Bhardwaj et al. demonstrated that the coronavirus nonstructural protein 15 (Nsp15) can interact with the retinoblastoma protein (pRb), a well-known tumor suppressor, leading to decreased overall levels of pRb.35 In a 2016 study, Ma-Lauer et al. reported that the coronavirus nonstructural protein 3 (Nsp3) stabilizes E3 ubiquitin ligase ring-finger and CHY zinc-finger domain-containing 1 (RCHY1), augmenting the RCHY1-mediated degradation of p53.36 Additionally, COVID‐19 has been associated with T‐cell depletion and activation of several other tumorigenic pathways, including JAK‐STAT, MAPK and NF‐κB.33
However, it is difficult to confirm the association between SARS-CoV-2 and hematologic malignancies through this case series alone. The patients had different clinical presentations of COVID-19, mainly concerning the severity of the viral infection; thus, we do not know at what level the host immune and inflammatory systems were affected. Another consideration is that the three cases occurred in a period close to the apex of the COVID-19 pandemic in Brazil, enhancing the possibility of the three occurrences being no more than casual. Despite the advances in the pathophysiological and clinical characterization of COVID-19, the future consequences of the pandemic to the population's overall health, including its burden on the incidence of hematologic diseases, are still to be elucidated. In this context, future dissection of the status of the local BM RAS in leukemogenesis is not only an academic issue, but also represents a clinically relevant basic research area.27
ConclusionIn conclusion, our report suggests some cases of hematologic malignancy in individuals who experienced COVID-19 months before the diagnosis could have been triggered by the local BM RAS imbalance induced by the SARS-CoV-2 infection. Considering the context of the pandemic, we understand the possibility that these are just coincidental phenomena. However, far from establishing a direct association between the conditions, the present case series aims instead to provide a hypothetical framework that stimulates further studies on the effects of SARS-Cov-2 on the local bone marrow renin-angiotensin system, foreseeing the potential consequences of the COVID-19 pandemic in leukemogenesis and in the incidence of hematologic neoplasms.
We would like to thank the patients for giving us verbal and written consent for the description of their cases.