Acute lymphoid leukemia (ALL) is a heterogeneous hematological neoplasm characterized by a proliferation of immature lymphoid cells. It is the most common form of childhood leukemia, but, in contrast, represents approximately 20% of all leukemias among adults.1,2
In these patients, as well as in those with other lymphoproliferative malignancies, immunosuppression related to the disease itself or to its treatment leads to a higher frequency of infectious complications.3,4 Varicella-zoster (VZV) and herpes-simplex virus (HSV) are among the most common infections in this scenario and are often associated to atypical clinical presentations, posing a diagnostic challenge and delaying adequate treatment.
We describe a case of an adult ALL patient with atypical, chronic, cutaneous lesions due to herpes vasculitis.
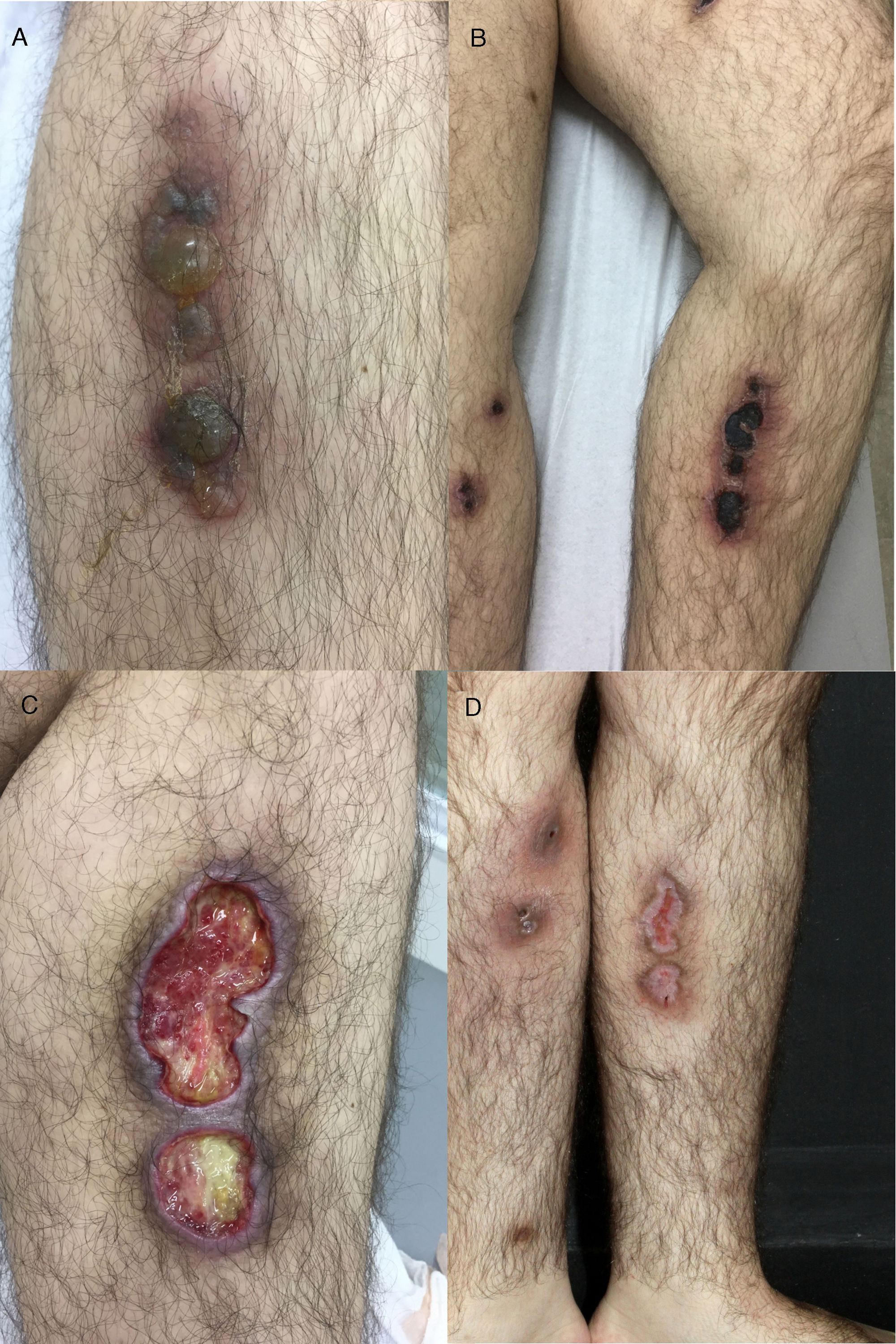
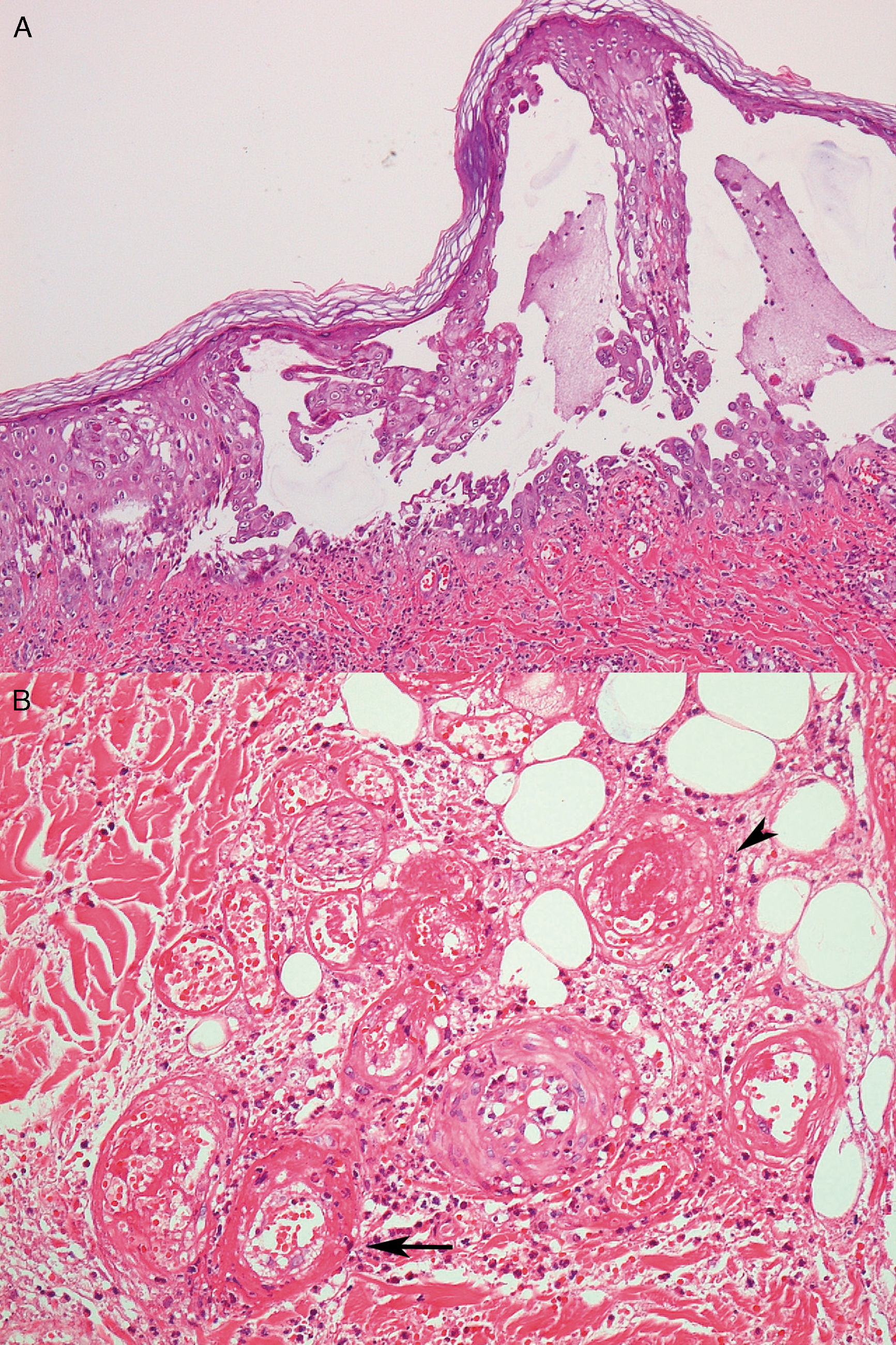
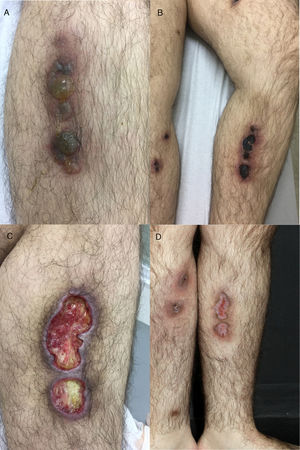
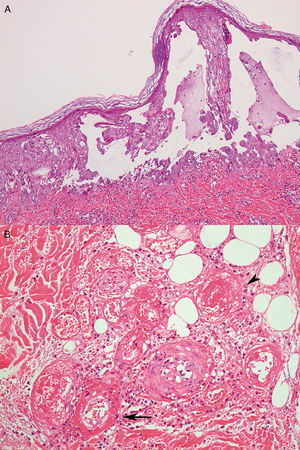
Case reportA 26-year-old male with a Philadelphia-negative ALL was treated with eight cycles of hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone). He had minimal residual disease negativity and was on maintenance therapy with dose-adjusted POMP regimen due to a prior grade 4 neutropenia (prednisone 200mg days 1–5, vincristine 2mg IV day 1, methotrexate 40mg IM weekly and 6-mercapthopurine 50mg orally days 1–28). Prophylaxis for pneumocystis (sulfametoxazol-trimetoprim), fungal infections (fluconazole) and herpes (acyclovir) was used during the induction phase and thereafter only while the patient was neutropenic during the first two cycles of maintenance phase. In the 24th cycle, he developed multiple tender, round, indurated deep nodules on both legs and thighs, with no history of ulceration and no systemic symptoms. He was treated with oral tetracyclines and topical antibiotics with the presumptive diagnosis of erythema nodosum; no improvement was seen. Two months later, the deep infiltrated lesions started to develop tense blisters on the surface (Fig. 1A). The patient had no signs of extracutaneous involvement. The complete blood count was normal (hemoglobin 13.7g/dL, leukocyte count 2×109L−1, neutrophil count 1.3×109L−1 and platelet count 153×109L−1). No imaging was done at the time. Since he was in apparent remission, it was important to exclude leukemic cutaneous infiltration and to differentiate from other dermatologic conditions such as neutrophilic dermatosis (Sweet syndrome or pyoderma gangrenosum), or atypical bacterial or fungal infection. Therefore, a punch biopsy was taken. Histology revealed an intraepidermal blister with viral cytopathic changes characteristic of herpes HSV/VZV infection (keratinocyte alterations with ballooning, acantholysis, marginalization of the nuclear chromatin and multinucleation; Fig. 2a). Leukocytoclastic vasculitis of the dermal vessels and vascular fibrinoid necrosis of the hypodermis could be seen; thrombosis and adipocyte necrosis were present (Fig. 2b). Viral culturing, staining with monoclonal antibodies and polymerase chain reaction (PCR) of skin and blood samples were not available to differentiate between HSV and VZV. Cytomegalovirus infection (CMV) was excluded due to the different cytopathic alterations related to this infection. Cultures for bacterial, mycobacterial and fungal infections were negative. Plasma PCR for HSV, VZV and CMV was not performed.
(A) Deep infiltrated lesions with tense blisters on the surface; (B) after starting therapy with acyclovir, lesions became necrotic and ulcerated covered with an adherent black eschar; (C) lesions evolved to extensive deep ulcerated areas; (D) three months after initiating therapy, almost complete healing with residual atrophic scars.
With these findings, a diagnosis of herpes vasculitis (either HSV or VZV) with thrombosis was made, and the patient was started on IV acyclovir 10mg/kg every 8h for 7 days, with maintenance with oral acyclovir 400mg three times daily thereafter. Some days after the initial therapy, lesions started to ulcerate, forming confluent necrotic plaques covered with an adherent black eschar which evolved to extensive deep ulcerated areas, as shown in Fig. 1B and C. Another biopsy was performed that showed extensive necrosis and thrombosis of larger vessels, with no signs of viral infection, so the patient was kept on acyclovir prophylaxis (400mg q12h) with complete healing and residual atrophic scars after eight months (Fig. 1D).
DiscussionClassically, cutaneous herpes virus lesions are described as painful, grouped vesicles on an erythematous base (in the case of herpes zoster, lesions follow a dermatome distribution).6 Lesions usually resolve after 7–14 days, even without treatment. Immunocompromised patients, e.g. those with HIV infection or neoplasia, especially hematological malignancies,7 tend to have a higher incidence of infections, with herpes being one of the most frequent. In these patients, lesions can become more invasive, might have a longer duration, with sometimes a chronic evolution and are an important cause of morbidity. They also have a greater potential for visceral dissemination and therefore, increased mortality.3–5
By histology, both herpes simplex and varicella zoster virus share similar aspects. Biopsy often reveals vesicles or ulcers with keratinocyte ballooning, acantholysis and necrosis with viral cytopathic changes such as multinucleated cells with nuclear molding.6 In the scenario of immunosuppression, atypical findings, such as dermal or deeper involvement, not only epidermal involvement might occur. Different techniques might be used to differentiate between herpes simplex (HSV) and varicella zoster virus (VZV) such as viral culturing, staining with monoclonal antibodies and PCR of biopsy specimens. Unfortunately, none of these were available for the case illustrated here, so the differentiation between HSV and VZV could not be made.
Cutaneous manifestations of CMV infection are not frequent. The cytopathic alteration related to CMV is the characteristic owl's eye intranuclear inclusion generally observed in endothelial cells and the pericytes of dermal vessels, not of keratinocytes. Thus, this differential diagnosis was ruled out based on the histological findings.
Many infections, including herpes, can trigger a vasculitis, more often due to an indirect mechanism where the agent stimulates an immune response against blood vessels, usually manifested as a palpable purpura. However, deeper and sometimes even obliterative vasculitis of larger vessels directly induced by the virus, as illustrated in the current case, are rare, with only a few similar cases reported in the literature.4,8,9
It is a matter of debate whether these atypical clinical and histological findings are related only to a more severe course, or, if the impaired cell-mediated immunity of these individuals, important for the local immune response against the agent, would cause a different path of dissemination and pathogenesis.4 Most of the times, after reactivation and spreading through the peripheral nerves, the virus primarily infects the epidermis with the main replication being within keratinocytes causing typical lesions and histological findings.10 The fact that, in our patient, lesions started as deep infiltrated nodules and only months later developed epidermal involvement goes in favor of this theory.
ConclusionThe current case demonstrates an example of a hematological patient, for whom the atypical presentation led to a delay in the correct diagnosis and, therefore, delayed the appropriate treatment. As the main limitation, we could not differentiate between herpes simplex and varicella zoster infection.
In conclusion, we highlight the importance of keeping herpetic infections on the differential diagnosis on immunosuppressed individuals, even when cutaneous lesions are not typical. An early and correct diagnosis and prompt treatment can avoid fatal outcomes related to viral dissemination.
Conflicts of interestThe authors declare no conflicts of interest.