As the Coronavirus Disease 2019 (COVID-19) pandemic unfolds around the world; answers related to the antibody response against the virus are necessary to develop treatment and prophylactic strategies. We attempted to understand part of the immune response of convalescent plasma donation candidates.
MethodWe carried out a cross-sectional, observational, non-intervention study, testing 102 convalescent plasma donation candidates for antibodies against the virus, relating these data to the time interval between symptom onset and sample collection, age, disease severity, and gender.
ResultsIn our sample, the individuals who developed a greater antibody response were the ones who had a longer time interval between symptom onset and sample collection, the ones who had been hospitalized and the subjects above 35 years old. Moreover, 17 individuals did not present any reactive antibodies.
ConclusionThese results are important in that they raise questions about the role of the humoral response against the virus, as some individuals do not develop antibodies to fight it. In addition, they help develop recruitment strategies for convalescent plasma donors, who should be asymptomatic for at least 21 days and are possibly more likely to have reactive antibodies after 35 days without symptoms.
In March 2020, the World Health Organization (WHO) declared the Coronavirus Disease 2019 (COVID-19) a pandemic.1 By May 2021, it had already infected more than 173 million people around the world.2
COVID-19 is a viral infection caused by SARS-CoV-2 that mainly induces fever, dry cough and fatigue. However, in serious cases, it may also lead to dyspnea, organ dysfunctions, seizures, pulmonary fibrosis and death.3 Worldwide, at the time this article was written, it had caused almost 3.7 million deaths4 worldwide and in Brazil alone, more than 474 thousand.4
Hence, finding a possible cure or efficient treatment has become one of the major concerns of scientists and researchers. The use of convalescent plasma (CP) may bring hope to critically ill patients, as this sort of therapy has been successfully used to treat hepatitis, measles, influenza, SARS-CoV, Ebola and other diseases.5
Antibody immunity against SARS-CoV-2 is still under debate. Some studies have shown that many individuals seroconvert 7 - 11 days after symptom onset6, but some subjects do not present reactive IgM or IgG levels at all.7 These events might suggest that not all individuals develop humoral immunity against the virus.
In Goiânia, a city in midwest Brazil, the infection and mortality rates of the disease have rapidly increased, posing a great threat to the population. Thus, the Hemocentro de Goiás (HEMOGO), the state public blood bank responsible for the collection and transfusion of blood and its components, started recruiting donors to collect CP, with the objective of transfusing it to critically ill patients hospitalized at affiliated hospitals.
ObjectiveThe purpose of this study was to analyze the antibody response in CP donors who had been previously tested for COVID-19 and who had recovered from the disease. Additionally, the antibody response according to the severity of the disease was analyzed. This analysis may allow an interpretation of how some people react to the virus, giving health workers tools to determine what kind of donors are more likely to be eligible to donate and contributing to a better comprehension of the immune process the patients undergo.
MethodThis was a non-interventional cross-sectional observational study carried out in Goiânia, Brazil from 26/6/2020 to 19/8/2020. The CP donation candidates were actively contacted by telephone by the Recruitment and Collection team of the HEMOGO. The list of convalescent subjects was provided by the State Department of Health.
The donor recruitment and eligibility were performed based on the protocol disseminated by the Food and Drug Administration (FDA).8 Subjects who were 18 to 60 years old, either men or nulliparous women, weighing more than 60 kg, and willing to donate were scheduled for screening procedures. They were required to present a diagnostic test for COVID-19 (either reverse transcription polymerase chain reaction (RT-PCR) or serological) and have been symptom-free for at least 14 days to be included in the initial part of the study. The specific day of symptom onset was verified for future correlation with other variables. During the appointment, they were submitted to a clinical interview and evaluated under the criteria imposed by the Brazilian Health Ministry.9 In this phase, they could possibly have been excluded, based on previous trips to endemic places, recent tattoo/definitive makeup procedures, multiple partners and others.
After the interview, the subjects who were eligible had nasopharynx swab samples collected to verify if they were negative for COVID-19 and proceeded to serology screening. The blood was submitted to chemiluminescence serology tests for sexually transmissible infections (STIs), hepatitis C, Chagas disease and HTLV 1 and 2. In addition, RT-PCR (using the XGEN MASTER COVID-19 kit), chemiluminescence immunoassays for SARS-CoV-2 IgM (using the MAGLUMI IgM 2019-nCoV assay) and IgG (using the Abbott Architect SARS-CoV-2 IgG assay) were also performed. The cutoff values for determining reactive antibody levels were in accordance with the test kit instructions.
The eligibility criteria for CP donors were IgG > 1.4 AU/mL, non-detectable SARS-CoV-2 RT-PCR and samples non-reactive to other diseases defined by Brazilian rules.9 Subsequently, the eligible subjects were scheduled to donate.
We sorted the individuals into three different groups, according to the severity of the disease, which was self-reported, as the main purpose of the original research was to recruit CP donors. Hence, the donors who had required hospitalization were placed in the “hospitalized” group, the individuals who had experienced symptoms (such as cough, anosmia and fever), but did not require hospital care, were placed in the “mildly symptomatic” group and those who had not experienced any symptoms were placed in the “asymptomatic” subjects.
All the results were analyzed, of both the eligible and the ineligible individuals, in search of an association between the severity of the disease and the development of IgM and IgG, a correlation between the antibody titer and the time interval between the onset of symptoms and the sample collection, including the severity of the disease and the age.
All the procedures were approved by, and performed in accordance with, the Research Ethics Committee (CAE: 31521820.3.0000.0035).
StatisticsData analysis was performed using the Stata software, version 15.0. Initially, the Kolmogorov-Smirnov test (K-S) with the Lilliefors correction was performed to verify the normality of the quantitative variables. Next, a descriptive analysis of the study variables was performed, with qualitative variables presented as absolute (n) and relative (%) frequencies. Quantitative variables were presented as median and interquartile ranges (IQR), in the absence of normality and in the mean and standard deviation (SD) in the presence of normality. 121
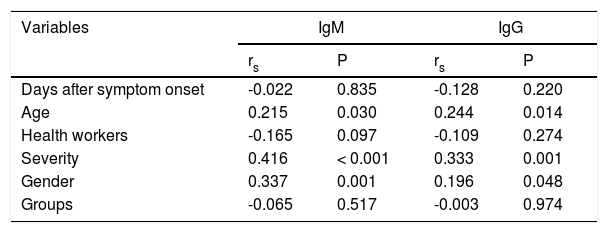
Subsequently, the Spearman's correlation analysis (rs) was performed between IgM and IgG levels and the study variables. The correlation coefficients were classified as no correlation (rs < 0.1), weak (rs = 0.1 - 0.3), moderate (rs = 0.3 - 0.5) and strong (rs > 0.5).11 In addition, the Mann-Whitney or Kruskal-Wallis test was used to compare qualitative variables that showed significance in the correlation test. Finally, multiple linear regression analysis was performed to verify the factors associated with the level of IgM and IgG antibodies in the sample (dependent variables). Only variables with p < 0.05 were included in the models in the previous analyses. The results of linear regressions were presented as a regression coefficient (β) and the respective 95%CI. Variables with the p-value < 0.05 were considered statistically significant.
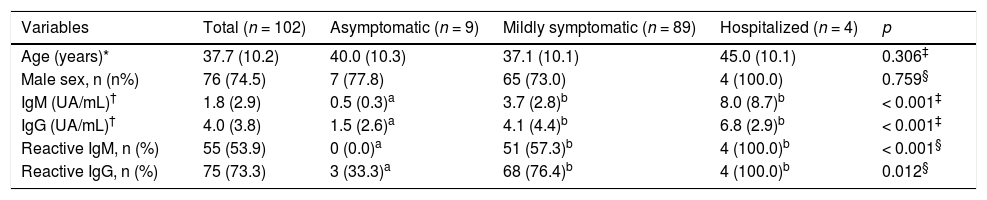
ResultsWe analyzed serological tests of 102 subjects during the study period. The descriptive analysis showed that 76 (74.5%) were men, the mean age was 37.7 years (SD: 10.2). The median between the symptom onset and the sample collection was 34 days (IQR: 16). The median IgM and IgG were 1.8 AU/ml (IQR: 2.9 AU/ml) and 4.0 AU/ml (IQR: 3.8 AU/ml), respectively (Table 1).
Main characteristics of the subjects of the study, according to the different severity groups.
| Variables | Total (n = 102) | Asymptomatic (n = 9) | Mildly symptomatic (n = 89) | Hospitalized (n = 4) | p |
|---|---|---|---|---|---|
| Age (years)* | 37.7 (10.2) | 40.0 (10.3) | 37.1 (10.1) | 45.0 (10.1) | 0.306‡ |
| Male sex, n (n%) | 76 (74.5) | 7 (77.8) | 65 (73.0) | 4 (100.0) | 0.759§ |
| IgM (UA/mL)† | 1.8 (2.9) | 0.5 (0.3)a | 3.7 (2.8)b | 8.0 (8.7)b | < 0.001‡ |
| IgG (UA/mL)† | 4.0 (3.8) | 1.5 (2.6)a | 4.1 (4.4)b | 6.8 (2.9)b | < 0.001‡ |
| Reactive IgM, n (%) | 55 (53.9) | 0 (0.0)a | 51 (57.3)b | 4 (100.0)b | < 0.001§ |
| Reactive IgG, n (%) | 75 (73.3) | 3 (33.3)a | 68 (76.4)b | 4 (100.0)b | 0.012§ |
Furthermore, after sorting them into groups, based on the severity of the disease, 89 subjects (87.3%) were in the mildly symptomatic group, 9 (8.8%) in the asymptomatic group, and 4 (3.9%) in the hospitalized group. In terms of reactive antibodies, 4 subjects (3.9%) had only reactive IgM, 24 (23.5%) had only reactive IgG, 41 (50%) had both reactive IgM and IgG and 23 individuals (22.5%) had no reactive antibodies.
The data presenting the main characteristics of the subjects of the study, according to the different severity groups, as well as the information found after the serology results were obtained, are described in Table 1.
In the asymptomatic group, in which individuals were submitted to the same tests as the mildly symptomatic and hospitalized individuals, 6 subjects did not present any reactive antibodies, and 3 presented only reactive IgG.
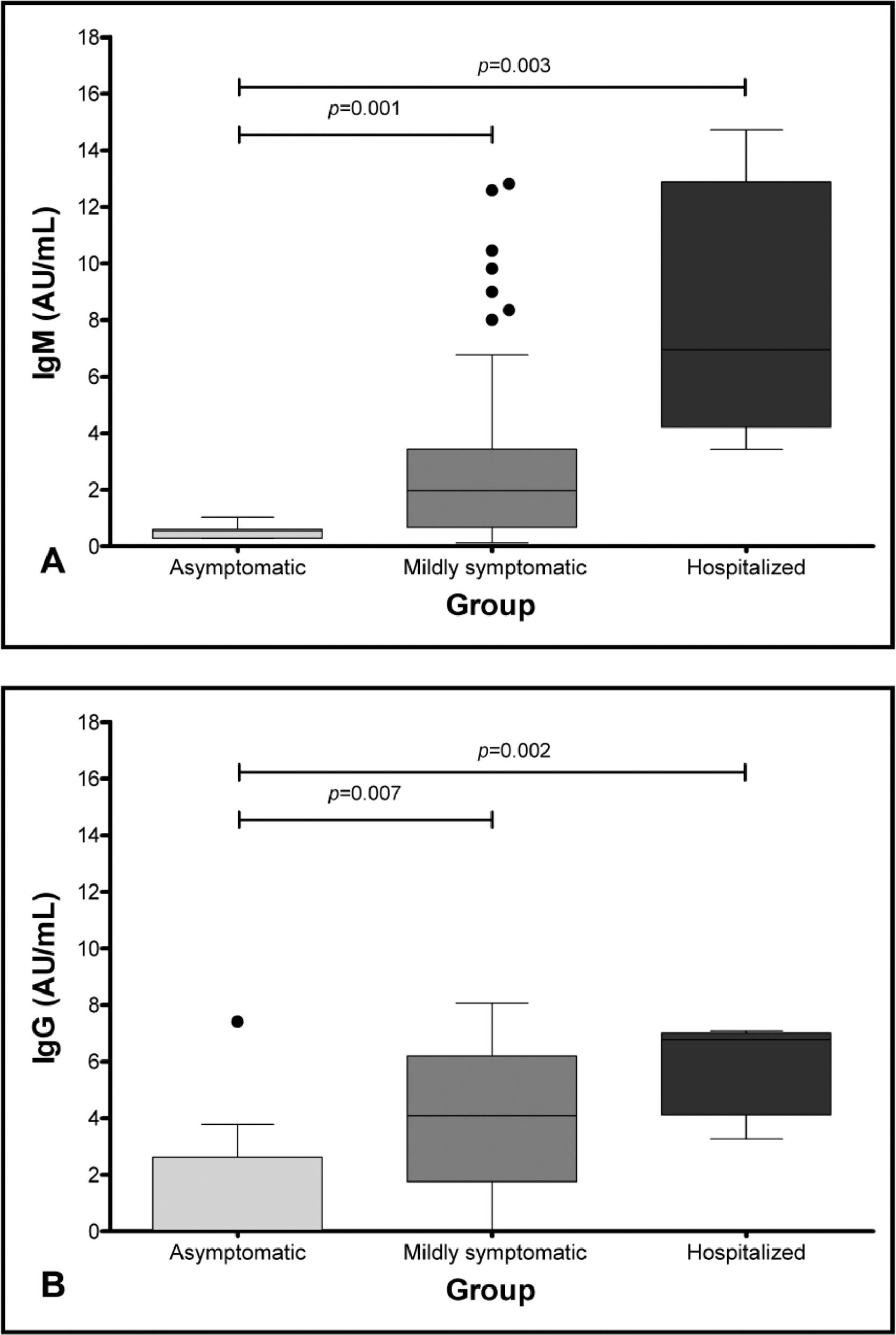
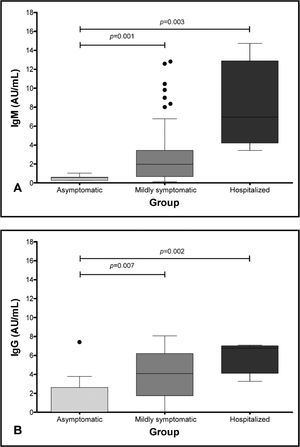
We observed that the median of IgM antibodies was statistically higher in the hospitalized group, when compared to the asymptomatic group (8.7 versus 0.5; p = 0.003), while the median in the mildly symptomatic group was higher than that of the asymptomatic group (3.7 versus 0.5; p = 0.001) (Table 1 and Figure 1A). Similarly, the median of IgG antibodies was higher in the hospitalized group, when compared to the asymptomatic group (6.8 versus 1.5; p = 0.002), and the median of IgG antibodies in the mildly symptomatic group was higher than in the asymptomatic group (4.1 versus 1.5; p = 0.007) (Table 1 and Figure 1B).
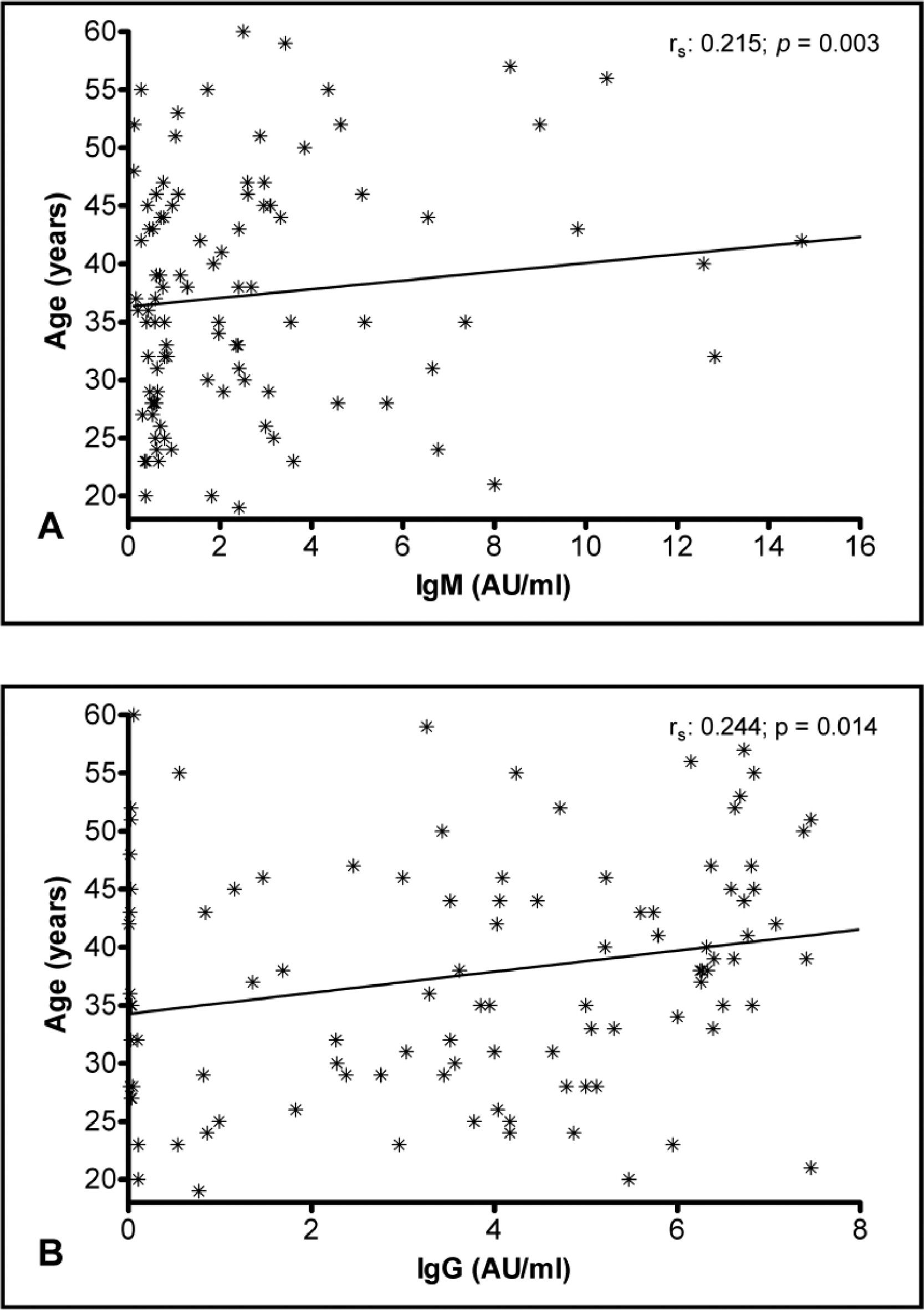
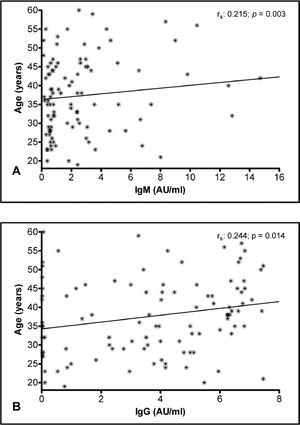
The multiple linear regression analysis was conducted to verify the relationship between severity and antibody levels after adjustment for gender and age as potential confounders. The analysis showed positive associations between the disease severity and antibody response. Furthermore, there was a positive association between age and levels of IgM and IgG antibodies (Table 2). This relationship can be seen in the correlation analysis shown in Figure 2A and B.
Correlation analysis between the variables and IgM / IgG levels.
rs=Spearman's correlation coefficient.
Moreover, persistent positive RT-PCR for SARS-CoV-2 was found in four (0.03%) male individuals, two of them in the 29 to 35-day group and two in the > 35-day group. Three of them were part of the mildly symptomatic group and one of them had been hospitalized. Also, in our analysis, all four individuals in the hospitalized group had both reactive IgG and IgM, one of them being part of the 29 to 35-day group and the remaining three, part of the > 35-day group.
DiscussionAntibody titerThe serology analysis, which was performed in this study, is an important tool to determine the number of people who were infected, so better targeted public policies can be created to estimate the number of people who have subclinical presentation of the disease10 and to find and recruit possible plasma donors who could contribute, either to the treatment of infected patients, or as post-exposure prophylaxis.5
In our sample, aiming to have a better idea of how the antibody response might be related to other factors, we sorted the individuals into the three aforementioned groups, in terms of the self-reported severity of the disease.
Some studies suggested that more severely affected patients can develop higher antibody levels.11,12 Long QX et al. studied 285 patients and found that the IgG levels were lower, with significant statistical difference, in the non-severe group after 14 days of symptom onset.11 Garcia-Beltran et al., studying 113 patients, also correlated disease severity with higher immunoglobulin levels.12 These results are similar to the ones we found in our analysis, when comparing the asymptomatic group to the mildly symptomatic group and the asymptomatic group to the hospitalized one (both showing a statistically significant difference, with p = 0.001 and p = 0.003, respectively) (Figure 1, Table 2).
Measuring the neutralizing antibodies (nAbs) levels is considered to be the best method to evaluate the protection against SARS-CoV-2, as they have the capacity to block the virus entry in cells and thus, prevent infection / disease.13,14 Because of this, those tests would be more suitable for the analysis of CP donor plasma.
At the same time, anti-nucleoprotein (anti-NP) assays are superior in the evaluation of early infection, as these proteins are more abundant in the viral structure. However, they correlate with less specificity and neutralizing ability15, which makes them less appropriate for CP donation. Unfortunately, neither the nAbs, nor the anti-NP tests were performed here, as these assays require specialized virology laboratories, which are not easily accessed in the region where the study was conducted.
Moreover, among the 102 subjects analyzed, only 3.9% presented positive RT-PCR for SARS-CoV-2. Their mean IgM and IgG were 4.15 and 4.52, respectively. These values are relatively higher than the mean values found in the asymptomatic and mildly symptomatic groups. Additionally, there was only one individual who had been hospitalized and still showed positive RT-PCR, who was part of the > 36-day group, and showed an IgM titer of 7.37 and an IgG level of 6.82. These values are higher than the mean value of the individuals in the same group. These findings, however, are in contrast with a study that analyzed persistent PCR positivity in patients16, which showed no correlation between persistent positive PCR and antibody strength. In this case, we must consider the fact that our sample is also small and that the analysis might not be completely consistent with reality.
We observed that 27/102 (26.5%) subject samples were non-reactive. Those who were tested up to the 21st day of the onset of the symptoms might not have had seroconversion yet. For those tested after the 28th day, we can infer that the antibodies had already been cleared. Some authors state that patients who had mild infections may react with fewer antibodies,17,18 which could explain this fact.
The IgM remained above 1.2 AU/mL in 55/102 donors (53.9%) after the 21st day of the symptom onset. Interestingly, 4 (7.2%) of these had only reactive IgM after the 29th day of the symptom onset, though most subjects who had reactive IgM after the 21st day of symptoms also had reactive IgG. We deduced that they were in a vigorous convalescence phase.
In addition, 75/102 (73.5%) subjects presented reactive IgG, regardless of the date of the symptom onset. Another fact worth mentioning is that 17 donors (16.6%) were IgM and IgG non-reactive 21 days after the onset of symptoms.
In this study, as we did not collect serial samples, we could not verify the average number of days for seroconversion to take place. Some authors recommend that a single collection should occur at least 21 days after the onset of symptoms, so seroconversion is observed.19 In our cohort, 3 (2.9%) donors had samples collected on the 21st day after the symptom onset. Of these, 2 had seroconversion with positive IgM and IgG and 1 had no reactive antibodies.
Antibody response and ageOur statistical analysis showed that the IgM and IgG titers were increasingly higher, according to the age of the individuals. Most of the studies that aimed to analyze the general antibody response and its association with age showed a decrease in the responsiveness of the adaptive immune system to newly encountered pathogens in individuals who are > 65 years old.20,21 Our study sample, however, consisted of individuals who are mainly 26 to 49 years old and thus, are not part of the population affected by the results of the mentioned papers. Moreover, they were part of a study concerned with collecting convalescent plasma.
Diversely, our findings are in accordance with a study that showed that the antibody levels, specifically against SARS-CoV-2 in the study population, adults ranging from 19 to 84 years old, were higher in older adults than in younger adults.22 They analyzed different anti-N and anti-S levels, finding that the older the individuals were, the more anti-N antibodies they developed. This may explain why older patients have worse outcomes than younger ones, as anti-N antibodies were found to predict poorer outcomes, probably because they are less protective than anti-S antibodies.23
Our paper, as several others,11,24,25 did not analyze the neutralizing activity of antibodies or their specificity. However, based on the aforementioned study,23 it is still possible to infer that the older individuals in our data did not necessarily mount a more robust response against the virus.
Antibody response and genderOur initial statistical analysis found that male subjects presented higher antibody titers than women, both for IgM (p = 0.001) and IgG (p = 0.049) (Figure 2). A study that analyzed the immunoglobulin titers according to gender during the first week of COVID-19 infection showed no statistically significant difference between males and females.26 Nevertheless, other publications have associated a more robust humoral response in female individuals than in male individuals facing viral infection.27,28
As our sample was mainly composed of male subjects, we can infer that this disparity may have created bias, with men showing a better antibody response than their female counterparts. Due to this fact, this result should not be extrapolated. Moreover, in the regression analysis, the correlation was not found.
ConclusionOur data analysis allowed us to verify that CP donation candidates who presented higher IgM and IgG titers were those who were tested more days after the symptom onset. Moreover, male individuals, those who were above 35 years old and those who had been hospitalized presented more antibodies than the other groups. Finally, 17 subjects did not present any reactive antibodies. Duly, studies that aim to focus on the humoral response against the virus would be valuable.
These results and the fact that this research is easily reproducible are the strong points of this study.
The limitations are related to the relatively small sample size, to the fact that we did not perform neutralization assays and to the disease severity being self-reported by the donation candidates. In addition, as we could not collect serial samples, we were not able to carry out a more accurate evaluation.
The authors thank Ana Cristina Mendes, Denyse Goulart, Ana Paula Santos for their technical support and José Cláudio Romero, head of the Instituto de Desenvolvimento Tecnológico e Humano (IdTech) for supporting the development of this project.