The COVID-19 pandemic in Mexico initiated the last week of February 2020.1,2 On March 17th,3,4 some public referral hospitals, including the National Institute of Medical Sciences and Nutrition “Salvador Zubiran” (INCMNSZ, for its acronym in Spanish), were shifted to “COVID-19-only centers” (“COVID-19-OC”). The objective of this study was to evaluate the modifications within the medical attention of hematopoietic cell transplantation (HCT) patients as a result of this pandemic-associated shift.
This study included and retrospectively analyzed 80 consecutive patients with scheduled HCT outpatient consultations, from March 17th to May 13th. Dates of appointments and patients’ data were obtained from the institutional electronic scheduling system and medical records (SoTeci). Categorical variables were described by frequencies and percentiles and continuous variables were described by the median and ranges. SPSS v.21 (IBM, Chicago, IL) was used to collect and analyze the data.
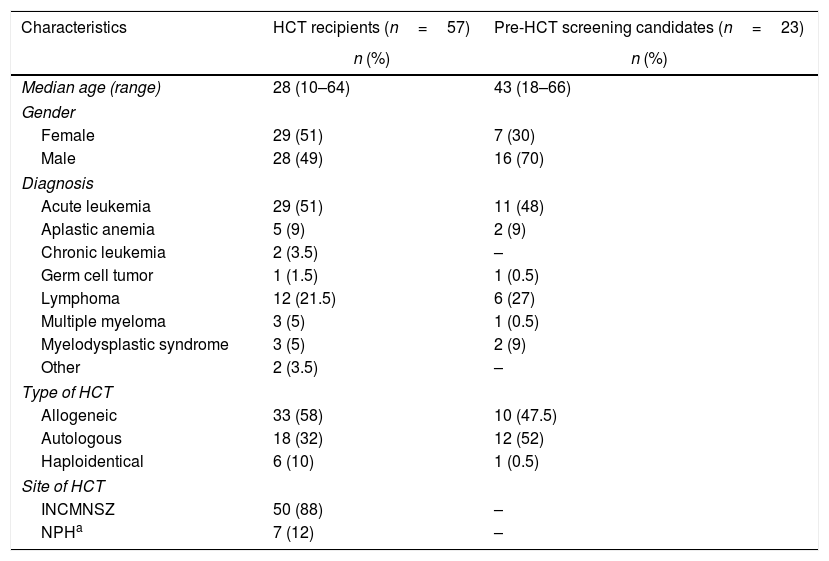
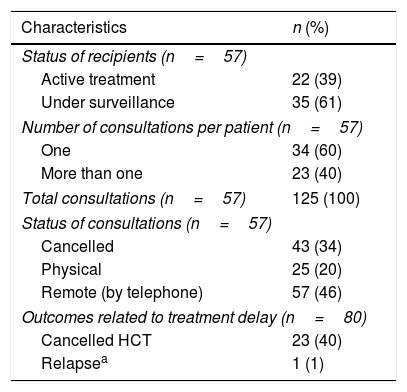
Fifty-seven patients had already received an HCT (recipients) and 23 were undergoing the pre-HCT screening (candidates). Overall demographics and characteristics are shown in Table 1. The most common underlying diseases among both, recipients and candidates were acute leukemias (51% and 48%, respectively). Table 2 shows the characteristics and modifications of the consultations of the recipients. Thirty-five (61%) recipients were under long-term surveillance without treatment and 22 (39%) were under active treatment. Overall, from the total 125 scheduled appointments, 46% were given remotely by telephone and only 20% were physical consultations. Forty-three (34%) appointments were cancelled, until further notice, as these recipients had received an HCT more than 2 years ago and did not have any complications during the last visits. None of the recipients undergoing consultations referred symptoms of COVID-19. On the other hand, from the 23 candidates, 65% were initiating the process (n=15) and 35% (n=8) had completed their screening. Outcomes as a result of treatment delay are also described in Table 2. All HCT had to be cancelled: 12 allogeneic, 10 autologous, and 1 haploidentical. During the 2-months period, only 2 candidates with acute leukemia required hospitalization; one due to relapse and COVID-19 diagnosis (admitted to the INCMNSZ), and the other had neutropenic fever (admitted to another center).
Demographics and characteristics of the HCT recipients and candidates (n=80).
| Characteristics | HCT recipients (n=57) | Pre-HCT screening candidates (n=23) |
|---|---|---|
| n (%) | n (%) | |
| Median age (range) | 28 (10–64) | 43 (18–66) |
| Gender | ||
| Female | 29 (51) | 7 (30) |
| Male | 28 (49) | 16 (70) |
| Diagnosis | ||
| Acute leukemia | 29 (51) | 11 (48) |
| Aplastic anemia | 5 (9) | 2 (9) |
| Chronic leukemia | 2 (3.5) | – |
| Germ cell tumor | 1 (1.5) | 1 (0.5) |
| Lymphoma | 12 (21.5) | 6 (27) |
| Multiple myeloma | 3 (5) | 1 (0.5) |
| Myelodysplastic syndrome | 3 (5) | 2 (9) |
| Other | 2 (3.5) | – |
| Type of HCT | ||
| Allogeneic | 33 (58) | 10 (47.5) |
| Autologous | 18 (32) | 12 (52) |
| Haploidentical | 6 (10) | 1 (0.5) |
| Site of HCT | ||
| INCMNSZ | 50 (88) | – |
| NPHa | 7 (12) | – |
INCMNSZ: National Institute of Medical Sciences and Nutrition “Salvador Zubiran”; NPH: National Pediatric Institute.
Characteristics of the HCT outpatient follow-up appointments and treatment delay outcomes (n=80).
| Characteristics | n (%) |
|---|---|
| Status of recipients (n=57) | |
| Active treatment | 22 (39) |
| Under surveillance | 35 (61) |
| Number of consultations per patient (n=57) | |
| One | 34 (60) |
| More than one | 23 (40) |
| Total consultations (n=57) | 125 (100) |
| Status of consultations (n=57) | |
| Cancelled | 43 (34) |
| Physical | 25 (20) |
| Remote (by telephone) | 57 (46) |
| Outcomes related to treatment delay (n=80) | |
| Cancelled HCT | 23 (40) |
| Relapsea | 1 (1) |
Pandemics, in this case as a consequence of the coronavirus SARS-CoV-2, involuntarily lead to changes within the healthcare systems.5 As the HCT ward at the INCMNSZ has four rooms, accounting the last 5 years, the mean number of HCT performed annually is 29. Nonetheless, our institution is one of the few public centers actively performing this procedure in adults from all the Mexican states.6,7 This year, only 6 HCT had been performed at our institution. Our 2-months results showed that from the recipients receiving consultations, most were by telephone under the circumstances of the benefit-risk equation to potentially develop COVID-19. More importantly, 23 candidates were not able to undergo an HCT, and from these, one relapsed. Those candidates could not be referred to other institutions, because even private hospitals, at least in Mexico City, the city where most centers performing this procedure are located, also cancelled this procedure since March. Only 2 centers performed urgent HCT among their patients. Nevertheless, at most centers, admissions were possible if required, contrary to our situation, were recipients could not be hospitalized but rather referred to another center, for which, thus far, there is a paucity of formal agreements. Fortunately, during the 2-months period, no post-transplant complications have been observed. In conclusion, we do not acknowledge until when, the shift at the INCMNSZ will prolong, but it is clear, that in the HCT scenario, the Mexican health system must be strengthened to meet the needs of all the HCT patients in the panorama of the current and future pandemics, as it has been reported that 6 HCT are performed per million inhabitants in our country7 due to paucity of centers performing this procedure (approximately 12), and consequently, although patient access is always considered, referrals are almost impossible.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Dr. Alberto Olaya-Vargas, Dr. Andres Gomez-de-Leon, Dr. Brenda Acosta-Maldonado, Dr. Guadalupe Rodriguez-Gonzalez, Dr. Martha Alvarado-Ibarra, Dr. Martha Gonzalez-Bautista, Dr. Myrna Pequeño-Luevano, Dr. Uendy Perez-Lozano, Dr. Yanet Ventura-Enriquez for their input regarding the status of HCT at their institutions.